Asia Pacific J Clin
Nutr (1997) 6(1): 68-71

Modulation of vascular endothelial
cell function by
palm oil antioxidants
MY Abeywardena1 PhD, RJ Head1
PhD, A Gapor2 MSc
1CSIRO, Division
of Human Nutrition, Kintore Avenue, Adelaide, SA 5000, Australia
2PORIM, Bandar Baru Bangi, 43000 Kajang,
Selangor, Malaysia.
Several cardiovascular risk factors including, hypercholesterolaemia
and hypertension, lead to diseased blood vessels due to endothelial
cell dysfunction. Recent studies also indicate that such alterations
in blood vessel function may involve free radical related mechanism(s).
Therefore, in the present study, two different preparations of palm
oils with variable antioxidant profiles, as well as a purified antioxidant
fraction extracted from unprocessed palm oil (tocotrienol-rich-factor;
TRF), were tested for their ability to influence blood vessel dysfunction
in the spontaneously hypertensive rat (SHR). Adult SHRs were fed
a synthetic diet supplemented (5% w/w) with either physically refined
palm oil (PO), golden palm cooking oil (Nutrolein; GPO) or olive
oil (OO; control diet). Antioxidant rich diet (TRF diet) was prepared
by supplementing the OO diet with 0.2% (w/w) TRF. After 12 weeks
of pre-feeding, segments of thoracic aorta were used to evaluate
vascular function. Compared to the normotensive Wistar-Kyoto (WKY)
control rats, aortic rings from the SHR showed impaired endothelium
dependent relaxation to acetylcholine (ACh) which was restored by
dietary TRF (p<0.05, ANOVA and Tukey’s test). In addition,
the paradoxical increase in tension in control hypertensive vessels
observed at higher doses of ACh was prevented by TRF and also by
the PO and GPO diets. Although the development of thromboxane-like
constrictor response, after the inhibition of nitric oxide in hypertensive
vessels, was unaffected by test diets, both TRF and GPO feeding
prevented the amplification of this unwanted constriction by a threshold
dose (7.2x10-10 M) of noradrenaline. Results suggest
a modulatory role for minor constituents of edible oils and are
in agreement with the recently reported benefits of natural antioxidants
against cardiovascular diseases.
Key words: endothelium, dietary
antioxidants, acetylcholine, spontaneously hypertensive rat
Introduction
The luminal surface of blood vessel is covered by
a monolayer of cells commonly referred to as the vascular endothelium.
The endo-thelium not only acts as a selective barrier against the
infiltration of various molecules into the underlying tissue, but
also modulates vascular tone, maintains cardiovascular homeostasis
and cell growth as well as inflammatory and immune responses in blood
vessels through the production of an array of both relaxant and constrictor
compounds1-3. The major endothelial cell derived relaxant
factors include; nitric oxide, prostacyclin, endothelium-derived hyperpolarising
factor and adenosine whilst thromboxane, free radicals, lipid hydroperoxides
and the vasoactive peptide endothelin are the main constrictor factors2-4.
Abnormalities in endothelial cell function therefore may promote vasospasm,
myocardial ischaemia, thrombosis, atherosclerosis or restenosis1-3.
Recent findings also suggest that endothelial function
is influenced by sustained hypertension, hypercholesterolaemia and
also by ageing1-5. Furthermore, data implies that an imbalanced
production of both the relaxant and constrictor factors may account
for the abnormal vasoconstriction observed in these disease conditions.
There is also an increasing body of evidence which indicates that
oxidative stress - oxygen derived free radicals and related products
- to be an important determinant in endothelial cell dysfunction6
and thus in the onset of several cardiovascular diseases.
Such findings implicate dietary antioxidants as well
as edible oils rich in endogenous antioxidants as potential candidates
to extend vascular protective actions. Indeed, several recent studies,
both in animal models and human subjects, have reported improvement
of blood vessel function by dietary antioxidant vitamin supplementation7-10.
Furthermore, a recent investigation in this laboratory also found
specific dietary n-3 polyunsaturated fatty acids11 and
several flavonoid compounds12 to offer vasoprotective actions
against the development of vascular dysfunction in the spontaneously
hypertensive rat.
Whilst it seems likely that cardiovascular benefits
of dietary n-3 polyunsaturates mediate through favourable changes
in the eicosanoid profile due to alterations in precursor/substrate
fatty acid availability11,13,14, it has also been reported
that eicosanoid production, hence cardiovascular function, may be
influenced by the non-fatty acid constituents in edible oils14,15.
In this context it can be speculated that palm oil, due to its fatty
acid composition (low polyunsaturated fatty acids) and high endogenous
antioxidant (tocotrienol and tocopherol) content14, may
be effective in influencing the peroxidation of membrane phospholipids
by oxygen radicals and may limit the consequent functional changes
of oxidative stress. In the newer preparations of palm oil (eg, golden
palm oil; Nutrolein), the carotenoids have also been preserved thus
improving the overall composition and content of endogenous antioxidants.
Therefore, in the present study two different palm
oil preparations with variable endogenous antioxidant profiles and
a purified fraction of palm oil antioxidants extracted from unprocessed
palm oil (tocotrienol-rich-factor; TRF), were evaluated for their
ability to influence vascular function in the spontaneously hypertensive
rat.
Materials
and methods
Animals
and diets
Four month old adult spontaneously hypertensive rats
(SHR; N=8 per group) and normotensive Wistar-Kyoto (WKY) control rats
were obtained from the colony established at this division. After
an equilibration period of two weeks, animals were fed ad libitum
a synthetic diet16, based on the American Institution of
Nutrition rodent diet (AIN-86). The total lipid content of the test
diets was 5% (w/w). Physically refined palm oil (PO), golden palm
cooking oil (Nutrolein; GPO) or olive oil (OO) served as the dietary
lipid source. The tocotrienol enriched diet was prepared by supplementing
the base diet with 0.2% (w/w) tocotrienol-rich-factor (TRF). The a-tocopherol level of the base diet was 0.04%
(w/w). Olive oil was the source of dietary lipid for the TRF supplemented
diet and the unsupplemented control diet. TRF and PO were supplied
by the Palm Oil Research Institute of Malaysia whilst GPO was kindly
provided by the Hai Loo Enterprise Sdn Bhd, (Johore Bahru, Malaysia).
OO was purchased locally.
Aortic
ring preparation
Upon completion of the feeding period, rats were stunned,
killed by decapitation and aorta from the thoracic region was carefully
excised and cleared of adhering tissue. Aorta was then cut into eight
rings, approximately 3 mm in length, and mounted under isometric conditions
at a resting tension of 4g in an organ bath chamber containing oxygenated
(95%/5% mixture of O2/CO2) Krebs-Henselit buffer
at 370C. The composition of the buffer solution was as
follows: (mM) 113 NaCl, 4.8 KCl, 1.2 KH2PO4,
1.2 MgSO4, 25 NaHCO3, 2.5 CaCl2,
11.2 glucose and ascorbic acid (0.57mM) in deionised water. The aortic
rings were allowed to equilibrate for 60 minutes before contracting
with KCl (20 mM) to test tissue viability. The increase in tension
was detected by Grass FT03 force transducers and recorded on a Graphtech
Linearecorder (FW33701) via an amplifier17.
Pharmacological
protocol
After establishing the tissue viability with KCl,
concentration response curves to noradrenaline (NA; 10-10 10-5
M) were constructed by cumulative additions to the bath. Vascular
relaxation to acetylcholine (ACh) was studied in tissues pre-contracted
with NA. In brief, after repeated washing and re-equilibration for
an hour, the rings were pre-contracted with NA (10-7 M)
before concentration response curves to ACh (10-9 -10-5
M) were constructed.
To study the spontaneous release of constrictor factor(s)
in hypertensive blood vessels12, several rings were incubated
with the inhibitor of nitric oxide (NO), Nw-Nitro-L-Arginine (NOLA;
10-4 M), for a period up to 60 minutes. Rings used for
these studies have not been exposed to noradrenaline prior to the
addition of NOLA. In some experiments, rings were pre-incubated with
a threshold concentration of NA (7.2x10-10 M) for 15 minutes
before the addition of NOLA.
Statistics
Contractile responses are expressed as % contraction
to KCl (20 mM) for each ring. Relaxation to acetylcholine are presented
as % contraction to 10-7 M to noradrenaline which normally
elicited a half-maximal response. Where appropriate, the results are
presented as Mean ± SEM. The means were compared with a one-way analysis
of variance (ANOVA) followed by Tukey’s test for multiple comparisons18.
A p value of <0.05 was considered as statistically significant.
Results
Table 1 shows the major fatty acids of dietary oil
supplements used in the present study. It is clear that the two palm
oil preparations differed in the proportions of palmitic (16:0), oleic
(18:1,n-9) and linoleic (18:2,n-6) acids. For example, physically
refined palm oil (PO) supplement contained a higher 16:0 (about 12%
higher) than golden palm cooking oil (GPO; Nutrolein) but a lower
proportion of monounsaturated 18:1, (n-9) and polyunsaturated linoleic
acid. In the GPO, this reduction in palmitic acid was offset by an
increase in oleic (7%) and also in linoleic acid. Olive oil was rich
in oleic acid (75%), and compared to the two palm oil supplements,
contained a lower proportion of 16:0.
The oil preparations also differed in their antioxidant
profiles. For instance, whilst both PO and OO supplements contained
no carotenoids, the GPO was rich in these antioxidants (approx. 450
ppm). However, the total vitamin E levels (tocopherol and tocotrienol
contents) were similar between the two palm oil samples and ranged
between 630-700 ppm. In contrast OO contained no tocotrienol, and
compared to palm oil, a lower tocopherol content (130 ppm).
Table 1. Major fatty acids of dietary oil supplements.
| |
Oil Supplement
|
| Fatty acid# |
OO |
PO |
GPO |
| 14:0 |
ND |
1.0 |
1.0 |
| 16:0 |
8.8 |
46.1 |
34.4 |
| 18:0 |
3.1 |
4.3 |
3.4 |
| 18:1 n-9 |
75.1 |
38.7 |
45.7 |
| 18:2 n-6 |
11.2 |
9.0 |
14.2 |
| 18:3 n-3 |
0.9 |
0.1 |
0.4 |
#Relative proportions of fatty acids are
expressed as % (w/w) of total fatty acids. Oil supplements are; OO
(olive oil); PO (palm oil) and GPO (golden palm cooking oil). Fatty
acids profiles were determined as reported previously14.
ND: not detected.
Figure 1. Restoration of impaired endothelium
dependant vascular relaxation in the SHR by dietary antioxidants.
Aortic rings were pre-contracted with noradrenaline (10-7M)
and exposed to increasing concentrations of acetylcholine. Data are
the mean ± SEM in preparations from 7-8 rats per group. Asterisk indicates
significant difference compared to hypertensive rats fed the control
diet (SHR-OO; p<0.05, ANOVA and Tukey’s test for multiple
comparisons). WKY(OO) - normotensive and SHR(OO) hypertensive - rats
fed the control (olive oil) diet. PO (palm oil); GPO (golden palm
oil) and TRF (tocotrienol-rich-factor) supplemented diets fed groups.
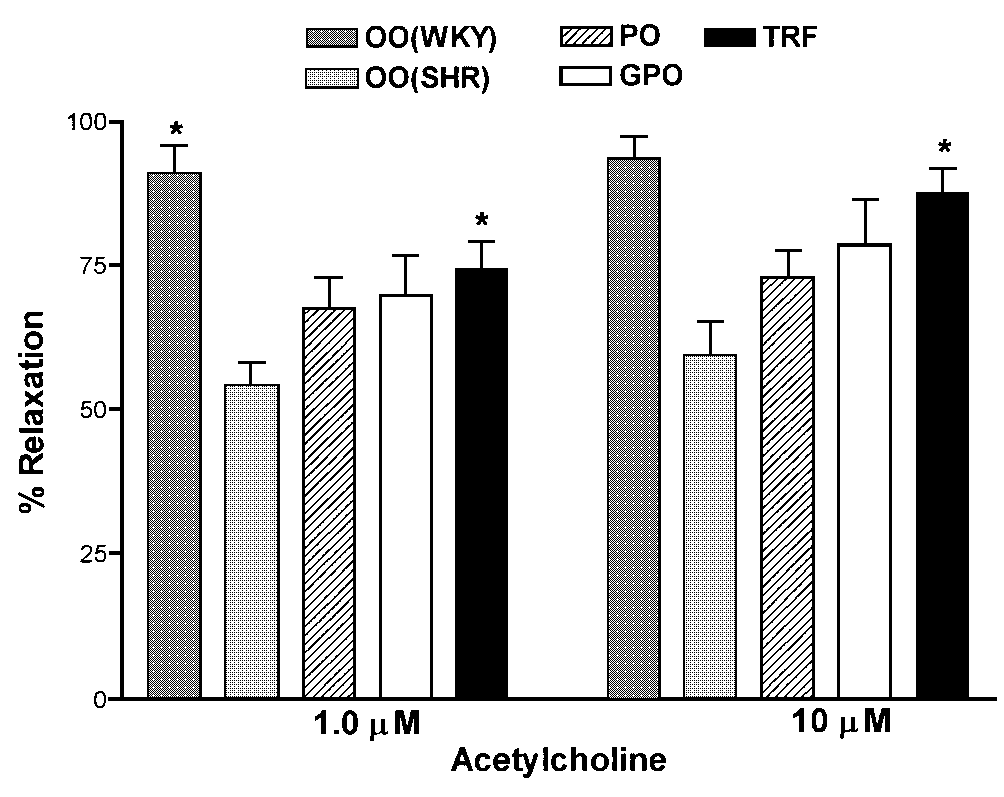
Figure 1 demonstrates the impaired vascular relaxation
to acetylcholine (ACh) in the spontaneously hypertensive rat. It is
clear that compared to the normotensive WKY control rats, the hypertensive
vessels relax only partially in OO rats. For example, the dose-dependant
increase in relaxation evident in the control vessels was not observed
with the diseased vessels and the maximal relaxation achieved at the
highest ACh dose amounted to only 59%. Incorporation of PO into the
diet tended to increase the relaxation response, to 67.7% and 72.9%
at 1 and 10 mM ACh respectively, but these changes failed to achieve
significance at the 5% level (Figure 1). A similar effect was observed
with the GPO diet fed animals. In contrast, TRF supplementation found
to be effective in restoring the impaired relaxation in hypertensive
vessels (p<0.05 vs SHR fed OO); over 87% relaxation was observed
at 10 mM ACh.
It was also found that ACh at higher doses (1-10 mM)
resulted in a paradoxical increase in contraction (30-40% of KCl response)
in vessels from SHR fed the control (OO) diet. In contrast, this response
was considerably reduced in animals fed different experimental diets.
For example, at the highest dose of ACh (10 mM) the contractions elicited
(expressed as % KCl contraction) were; PO 9.8±2.1*; GPO
6.4±1.7*; TRF 2.1±0.4* (* indicates significant
difference; p<0.05 vs OO).
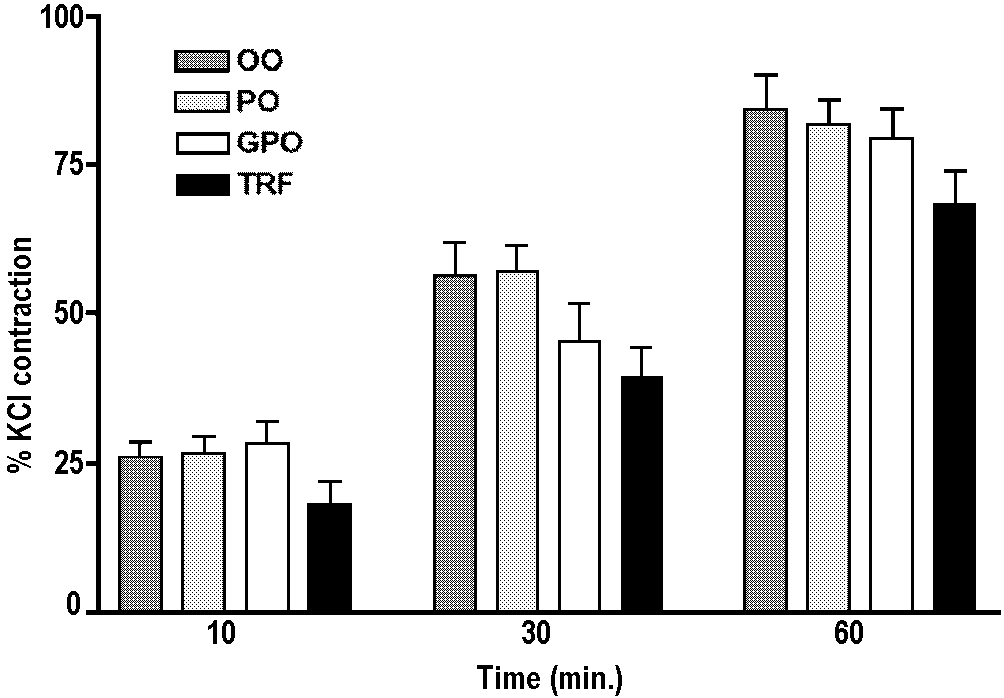
Figure 2 shows the time dependant release of constrictor
factor(s) from the blood vessels after the inhibition of nitric oxide
(NO) with Nw-Nitro-L-Arginine (NOLA; 10-4 M). It is clear that the release
of the constrictor factor(s) is a slow process as there appears to
be a gradual rise in tension with time. This increase in contraction
normally tends to plateau 45 minutes after the inhibition of NO (data
not shown). None of the treatments was found to significantly modify
this constrictor response in hypertensive vessels (P>0.05, ANOVA
and Tukey’s test for multiple comparisons). TRF dietary rats
however, displayed the lowest mean levels for all time points studied.
Compared to hypertensive rats, the constrictor response was virtually
absent in the normotensive control animals. For example, at 60 minutes
the NOLA induced contraction was only 2.2% of the KCl contraction
(data not shown).
Figure 2. Abnormal thromboxane-like constrictor
response in the SHR. After contraction with KCl (20 mM), aortic rings
were incubated with an inhibitor of endothelial cell nitric oxide,
Nw-Nitro-L-Arginine (NOLA; 10-4M), for a period up to 60 minutes,
to unmask the release of constrictor factor(s). The tension developed
is expressed as a percentage of maximal contractile response to KCl.
Results are the mean ± SEM; p>0.05.
It was also found (Figure 3) that the release of this
constrictor factor(s) after inhibition of nitric oxide with NOLA was
considerably potentiated by the presence of a threshold dose (7.2x10-10
M) of noradrenaline (NA). For example, in the control SHR fed the
olive oil diet, presence of this low dose of NA resulted in over 80%
increase in the constriction at the 10 minute (Figure 3) and over
30% at the 30 minute time point following the inhibition of nitric
oxide. This amplification of the NOLA induced unwanted constriction
by NA was prevented by both TRF and GPO supplementation of the diet.
Discussion
The present study supports several recent investigations
which have demonstrated that blood vessel function can be influenced
by dietary lipids and antioxidants7,8,11. For instance,
natural antioxidants vitamin E and b-carotene have recently been shown
to restore the impaired vascular relaxation observed in hyper-cholesterolaemia
and atherosclerosis7-10. Similarly, vitamin E and several
plant flavonoid compounds have also been found to exert vasoprotective
actions in hypertensive vessels7,12. In the present investigation,
it was found that tocotrienol-rich-factor (TRF), extracted from palm
oil, mimicked the previously reported14 effects of vitamin
E (a-tocopherol) and restored the impaired vascular relaxation in the spontaneously
hypertensive rat (SHR). Both PO and GPO feeding also displayed increased
relaxation to ACh compared to the control group, although this failed
to reach statistical significance. Taken together, these findings
tend to imply that the improvement of vascular relaxation in this
model is likely to be mediated through the antioxidant components
rather than the fatty acid constituents of edible oils. Both PO and
GPO were rich in natural antioxidants whilst OO was found to contain
a lower vitamin E content. Data also indicate that endogenous antioxidant
content of edible oils alone may not be sufficient to fully restore
the impaired vascular function in diseased vessels.
Figure 3. Effect of dietary antioxidants on
noradrenaline potentiation of abnormal constrictor response. Blood
vessels were pre-incubated with a threshold dose of noradrenaline
(7.2x10-10M) before unmasking of the spontaneous constriction
with NOLA (10-4M). Asterisk indicates significance at the
5% level (N=7 per group). Both TRF and GPO supplementation prevented
the amplification of tension development by noradrenaline. (r ) basal release; (n ) in the presence of noradrenaline.
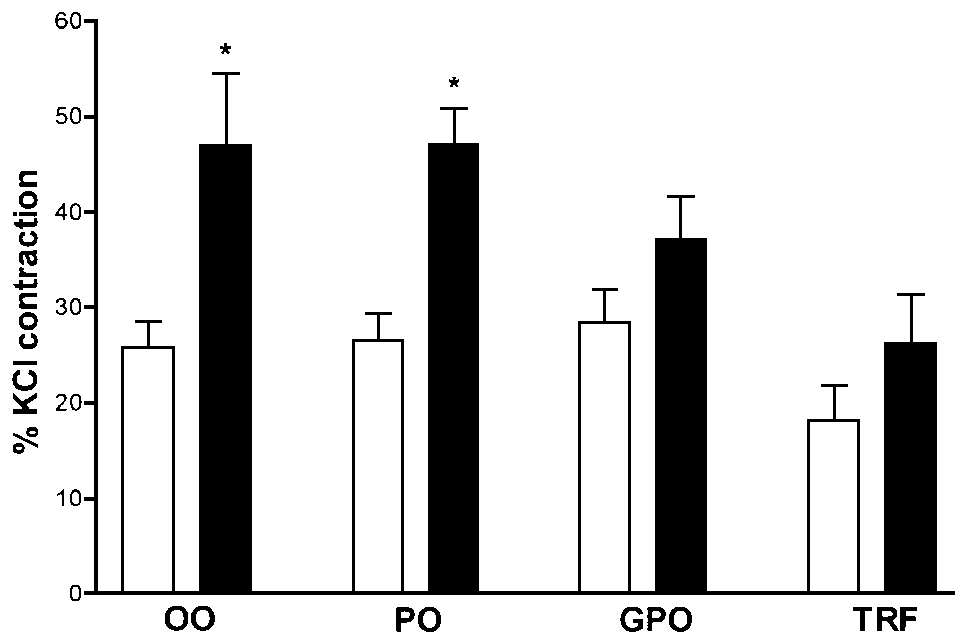
As in the case with the TRF supplemented diet, both
PO and GPO groups prevented the paradoxical increase in contractions
to ACh which occurred mainly at the upper end of the dose-response
curve. Such ACh induced contractions have previously been observed
in blood vessels from the SHR and are thought to be due to the release
of endothelium derived contracting factors (EDCFs; 3,4). The candidate
mediators include prostaglandin H2 (PGH2) and
oxygen derived free radical superoxide anion4,19. The ability
of antioxidant rich diets to prevent the formation of EDCFs tends
to imply a role for superoxide anion in mediating the ACh induced
constriction. Furthermore, the effectiveness of antioxidant supplementation
in restoring the endothelium dependent vascular relaxation to ACh,
reported prev-iously7-9 and also observed in the present
investigation, implies that a free-radical related mechanism is involved
in causing vascular dysfunction in the SHR. This speculation is further
supported by recent reports which suggest that in this model of hypertension
(SHR) the main endothelium derived relaxing factor pathway (L-arginine/nitric
oxide) to be functioning normally2,4,20.
In the present study, the thromboxane-like constrictor
response which is evident only after the inhibition of endothelial
cell NO, was not influenced by antioxidant or edible oil supplementation
of the diet. However, GPO diet as well TRF feeding prevented the amplification
by a low dose of NA of this NOLA induced constriction. The mechanism
responsible for this action is not clear, but parallel studies with
several pharma-cological tools (MY Abeywardena, unpublished observations)
suggest an involvement of eicosanoid metabolites in this process.
In this regard it is also worth noting that minor constituents in
dietary palm oil have previously been reported to modulate eicosanoid
biosynthesis in cardiac muscle14,21. In addition, our recent
findings suggested that the nature of this unwanted constriction to
be a prostaglandin intermediate such as PGH212
further strengthening the above speculation of a possible modulation
of eicosanoid metabolism in blood vessels by dietary antioxidants.
In conclusion, the findings of the present study are
in agreement with recent reports which assign a beneficial role for
natural antioxidants against cardiovascular diseases22-24.
Acknowledgments. This study was supported in part by a grant from the Palm Oil
Research Institute of Malaysia. We thank M Adams for the preparation
of diets and care of the animals.
References
- Rubanyi GM. The role of endothelium in Cardiovascular
homeostasis and diseases J Cardiovasc Pharmacol 1993; 22: S1-S14.
- Luscher TF, Noll G. Endothelium dysfunction in
the coronary circulation. J Cardiovasc Pharmacol 1994; 24: S16-S26.
- Furchgott RF, Vanhoutte PM. Endothelium-derived
relaxing and contracting factors. FASEB J 1989; 3: 2007-2018.
- Luscher TF, Boulanger CM, Dohi Y, Yang Z. Endothelium-derived
contracting factors. Hypertension 1992; 19: 117-130.
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita
Y, Fujishima M. Age and hypertension promote endothelium-dependent
constrictions to acetylcholine in the aorta of the rat. Hypertension
1989; 14: 542-548.
- Stam H, Hulsmann, WC, Jongkind, JF, Van der Kraaij,
AMM, Koster, JF. Endo-thelial lesions, dietary composition &
lipid peroxidation. Eicosanoid 1989;2:1-14.
- Abeywardena MY, Head RJ. Modulation of blood vessel
function by dietary anti-oxidants in the spontaneously hypertensive
rat. In Ong ASH, Packer L, eds. Nut-rition, lipids, health and disease.
AOCS Press, Champaign, IL, 1995: 139-150.
- Keaney JF, Gaziano JM, Xu A, Frei B, Curran-Celentano
C, Shwaery GT, Loscalzo J, Vita JA. Dietary antioxidants preserve
endothelium-dependent vessel relaxation in cholesterol-fed rabbits.
Proc Natl Acad Sci 1993; 90: 11880-11884.
- Stewart-Lee AL, Forster LA, Nourooz-Zadeh J, Ferns
GAA, Anggard EE. Vitamin E protects against impairment of endothelium-mediated
relaxations in cholesterol-fed rabbits. Arterioscler Thromb 1994;
14: 494-499.
- Levine GN, Frie B, Koulouris SN, Gerhard MD, Keaney
JF, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction
in patients with coronary artery disease. Circulation 1996; 93:
1107-1113.
- McLennan PL, Howe PRC, Abeywardena MY, Muggli R,
Raederstorff D, Mano MT, Rayner T, Head RJ. The cardiovascular protective
role of docosahexaenoic acid. Eur J Pharmacol 1996; 300: 83-89.
- Abeywardena MY, Head RJ. Vascular protective actions
of natural antioxidants. In: Natural antioxidants : Molecular mechanisms
and health effects. AOCS Press, Champaign, IL, 1996: 584-594.
- Abeywardena MY, McLennan PL, Charnock JS. Differential
effects of dietary fish oil on myocardial prostaglandin I2
and thromboxane A2 production. Am J Physiol 1991; 260:
H379-385.
- Abeywardena MY, Charnock JS. Dietary lipid modification
of myocardial eicosa-noids following ischaemia and reperfusion in
the rat. Lipids 1995; 30: 1151-1156.
- Gutfinger T. Polyphenols in olive oil. J Am Oil
Chem Soc 1981; 58: 966-968.
- Rayner TE and Howe PRC. Purified w-3 fatty acids
retard the development of proteinuria in salt-loaded hypertensive
rats. J Hypertens 1995; 13: 771-781.
- Dyer SM, Taylor DA, Bexis S, Hime NJ, Frewin DB,
Head RJ. Identification of a non-endothelial cell thromboxane-like
constrictor response and its interaction with the renin-angiotensin
system in the aorta of spontaneously hypertensive rats. J Vasc Res
1994 31:52-60.
- Wallenstein S, Zucker CL, Fleiss JL. Some statistical
methods useful in circu-lation research. Circulation 1980; 47: 1-9.
- Jameson M, Dai FX, Luscher T, Skopec J, Diederich
A, Diederich D. Endothelium-derived contracting factors in resistance
arteries of young spontaneously hypertensive rats before development
of overt hypertension. Hypertension 1993; 21: 280-288.
- Luscher TF, Vanhoutte PM. Endothelium-dependent
contractions to acetylcholine in the aorta of the spontaneously
hypertensive rat. Hypertension 1986;8: 344-348.
- Abeywardena MY, McLennan PL, Charnock JS. Changes
in myocardial eicosanoid production following long-term dietary
lipid supplementation in rats. Am J Clin Nutr 1991; 53: 1039S-1041.
- Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. The
antioxidant vitamins and cardiovascular disease. Ann Intern Med
1995; 123: 860-872.
- Hertog MGL, Feskens EJM, Hollman, PCH, Katan, MB,
Kromhout D. Dietary antioxidant flavonoids and risk of coronary
heart disease: the Zutphen elderly study. Lancet 1993; 342: 1007-1011.
- Williams RJ, Motteram JM, Sharp CH, Gallagher PJ.
Dietary vitamin E and the attenuation of early lesion development
in modified Watanabe rabbits. Atherosclerosis 1992; 94: 153-159.
Modulation of vascular endothelial
cell function by palm oil antioxidants
MY Abeywardena, RJ Head, A Gapor
Asia Pacific Journal of Clinical Nutrition (1997) Volume 6, Number
1: 68-71


Copyright © 1993 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
 to the top
to the top