1000
Asia Pacific J Clin Nutr (1997) 6(4): 273-276
Asia Pacific J Clin
Nutr (1997) 6(4): 273-276

Effects
of childhood malnutrition on Insulin-like Growth Factor-l (IGF-I)
and IGF-Binding Protein-3 levels: a Malaysian and New Zealand analysis
Wan Nazaimoon WM1, PhD, Rahmah R2,
MMed (Paed),
Osman A2, PhD, Khalid BAK2,
FRACP, PhD,
Livesey J3, PhD
1Division of Endocrinology,
Institute for Medical Research, Jalan Pahang, Kuala Lumpur, Malaysia
2Department of Medicine, Faculty of Medicine,
Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
3Department of Endocrinology, Christchurch
Hospital, Christchurch, New Zealand
Plasma IGF-I and IGFBP-3 levels were measured in
190 clinically confirmed malnourished Malaysian children and 86
normal New Zealand children. Children were grouped into age groups
of 4-10 and 11-15 years, and were classified as normal, moderate
and severely stunted using WHO criteria. Plasma IGF-I levels of
moderate and severely stunted children were significantly lower
than age-matched normal children, in the 4-10 (p<0.01 and p<0.001
respectively) and 11-15 (p<0.01 and p<0.001 respectively)
years age groups. Malnourished children of age group 4-10 years
had significantly lower (p<0.001) IGFBP-3 levels compared to
normal but in the older group, significant difference was observed
only in the severely stunted children. Compared to those who were
moderately stunted, IGF-I and IGFBP-3 levels of severely stunted
children were significantly lower (p<0.001 and p=0.03 respectively).
There was significant increase in IGF-I and IGFBP-3 levels between
age groups, in normal (p<0.001 and p=0.02 respectively), moderate
(p<0.01 for both) and severely (p<0.001 and p<0.001 respectively)
stunted children. Significant correlation between IGF-I and IGFBP-3
levels was observed both in normal (r=0.511, p<0.001) and malnourished
(r=0.657, p<0.001) children. Standard deviation score (SDS) of
height and weight correlated significantly to the IGF-I levels,
both in age group of 4-10 (r=0.472, p<0.001 and r=0.443, p<0.001
respectively) and 11-15 years (r=0.445, p<0.001 and r=0.539,
p<0.001 respectively). Correlations between SDS of height and
weight and IGFBP-3 levels were highly significant only in the younger
children (r=0.494, p<0.001 and r=0.489, p<0.001 respectively).
The study showed that nutrition exerts a greater effect on IGF-I
compared to IGFBP-3, suggesting that its significance is in determining
the linear growth of malnourished children.
Key words: malnutrition, childhood,
Malaysia, New Zealand, growth, height, weight, Insulin-like Growth
Fact 1000 or-l (IGF-I), IGF-Binding Protein-3
Introduction
Nutrition is an important determining factor for optimal
growth and development in children. Studies conducted under the Nutrition
Collaborative Research Support Pro-gram showed that mild to moderate
malnutrition leads to stunting of growth and by the age of 3 to 4
months, children suffer permanent losses in their ability to grow
and develop normally1.
Malnutrition induces a state of growth hormone (GH)
resistance. The mechanisms involved depend on the severity, duration
and time of onset, postulated to be due to down-regulation of GH-receptors
or defects at the post-receptor level2. However, as the
growth-promoting effect of GH is mediated in part by insulin-like
growth factor-I (IGF-I)3, the low levels of this growth
factor and its carrier protein, IGF binding protein-3 (IGFBP-3), during
nutritional deprivation2,4,5 have also been implicated
as contributing factors for GH insensitivity. To further understand
the possible alterations and derangement involved, we undertook this
study to evaluate the effect of childhood malnutrition on IGF-I and
IGFBP-3 levels and their associations to height attainment, in comparison
with age-matched apparently healthy children.
Methods
This is a collaborative study between 3 centres; 190
mild to moderately malnourished children were recruited from 3 Malaysian
villages of low socioeconomic status, while, for comparison purposes,
86 healthy children, without limitation in food intake, were recruited
by the Christchurch Hospital, New Zealand. The children were between
4 and 15 years old, thoroughly examined and malnutrition con-firmed
clinically by the Paediatric Endocrinologist involved in the study.
Blood was collected from the forearm vein and plasma aliquots were
immediately stored at -40°C until assayed for IGF-I and IGFBP-3. Parents
gave informed consent prior to the study and the protocol was approved
by the Ethics Committee of the Medical Faculty, Universiti Kebangsaan
Malaysia and the Southern Regional Health Area Ethics Committee, New
Zealand. Standard deviation scores (SDS) for weight and height for
chronological age were calculated using CDC Anthropometric Software
Package.
Plasma IGF-I and IGFBP-3 were quantitated by RIA using
kits purchased from Nichols Institute Diagnostics (San Juan Capistrano,
CA). An acid-ethanol extraction procedure was used for the IGF-I assay.
Intra- and inter-assay coefficients of variations were 5.8 and 9.1%
respectively for IGF-I and 4.9 and 8.4% respectively for IGFBP-3.
Assay sensitivity was 20ng/ml for IGF-I and 0.13m g/ml for IGFBP-3.
Statistics
Data were analysed according to 2 age-groups: 4-10
and 11-15 years. Differences between normal and malnourished, and
between age groups were analysed by the non-parametric Wilcoxon test.
Associations between IGF-I or IGFBP-3 and weight or height SDS were
determined by Spearman correlation. Results are expressed as mean
± SEM.
Figure 1. Plasma IGF-I levels (mean ± SEM) in normal, moderate and severely
stunted children of 4-10 and 11-15 years age group.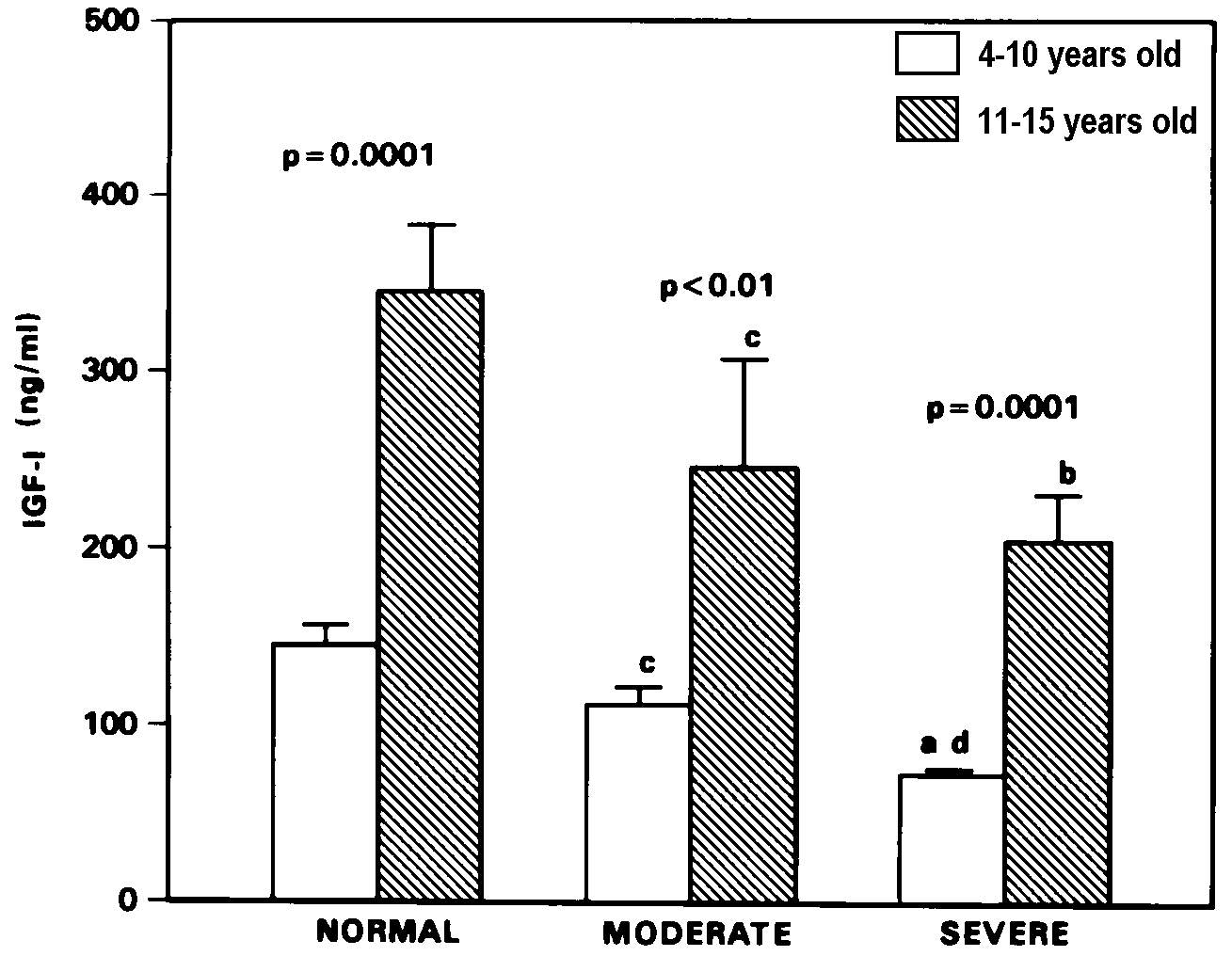
Significance of difference between age groups is shown
by p value above each histogram pair for level of severity of stunting.
ap=0.0001, bp<0.001, cp<0.01
compared to 1000 age-matched normal children. dp<0.001
compared to age-matched moderately stunted children.
Results
Based on WHO definition1,6, the malnourished
children were classified as moderate stunting when their computed
height SDS values were between 2 and 3 SD below the reference median,
and as severe stunting when the height SDS values were more
than 3 SD below the reference median. The median and 95% range for
height and weight SDS of both the malnourished and normal statured
children are presented in Table 1. Plasma IGF-I levels of moderate
and severely stunted children were significantly lower than age-matched
normal children, both in the 4-10 (p<0.01 and p=0.0001, respectively)
and 11-15 (p<0.01 and p=0.0001 respectively) years age groups (Figure
1). Similarly, compared to normal, IGFBP-3 levels of the 4-10 years
old moderate and severely stunted children were low (p = 0.0001) (Figure
2). In the older age group however, significant decrease in IGFBP-3
levels was observed only in the severely stunted group (p<0.01
versus age-matched normal). As shown in Figures 1 and 2, the IGF-I
and IGFBP-3 levels of the 4-10 years old severely stunted children
were significantly lower (p=0.0005 and p=0.03 respectively) than those
who were only moderately stunted. Nevertheless, there was significant
increase in IGF-I and IGFBP-3 levels with age in the moderate (p <
0.01 for both) and severely (p = 0.0001 and p<0.001 respectively)
stunted children, comparable to that observed in normal statured children
(p=0.0001 and p=0.02 respectively).
Table 1. Characteristics of normal, moderate
and severely stunted children.
| |
4-10 years old
|
11-15 years old
|
| |
Normal
|
Moderate
|
Severe
|
Normal
|
Moderate
|
Severe
|
| |
(n=56)
|
(n=60)
|
(n=76)
|
(n=30)
|
(n=14)
|
(n=40)
|
| Height |
-0.01
< 1000 /td>
|
-2.51
|
-3.77
|
+0.05
|
-2.41
|
-3.76
|
| SDS |
-1.2 - +2.0
|
-3.0 - -2.1
|
-5.7 - -3.1
|
-1.7 -+2.3
|
-3.0 - -2.1
|
-5.1 - -3.1
|
| Weight |
+0.39
|
-2.21
|
-3.0
|
+0.33
|
-2.2
|
-2.74
|
| SDS |
-1.2 - +3.0
|
-3.5 - -1.4
|
-4.1 - -1.9
|
-1.5 -+1.4
|
-3.0 - -1.9
|
-3.8 - -1.4
|
Values are the median and 95% range.
Figure 2. Plasma IGFBP-3 levels (mean + SEM)
in normal, moderate and severely stunted children of 4-10 and 11-15
years age group.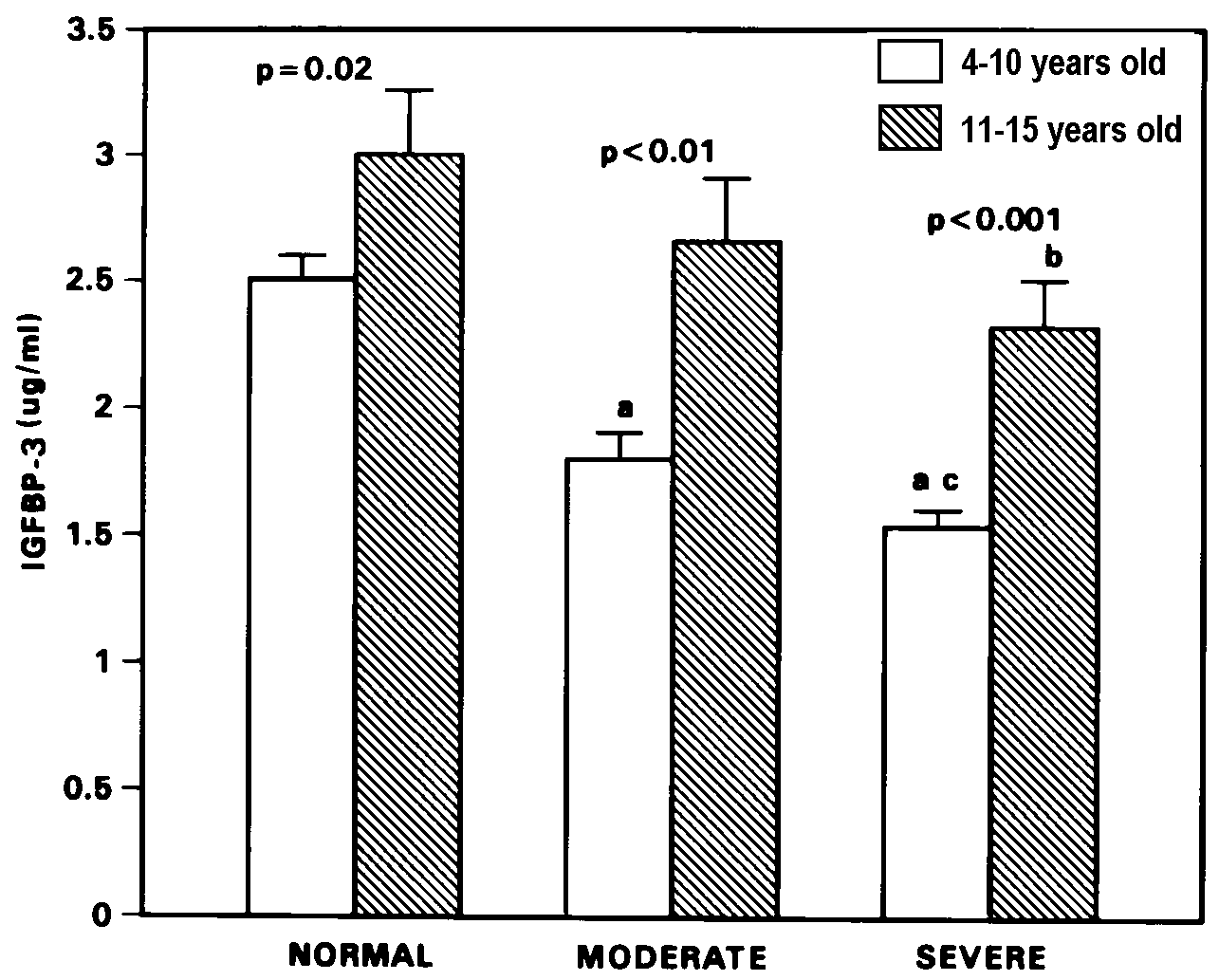
Significance of difference between age groups is shown
by p value above each histogram pair for level of severity of stunting.
ap=0.0001, bp<0.01 compared to age-matched
normal children. cp=0.03 compared to age-matched moderately
stunted children.
Correlation between IGF-I and IGFBP-3 levels was highly
significant, both in the normal (r=0.511, p=0.0001, Figure 3) and
malnourished children (r=0.657, p=0.0001, Figure 4). The associations
between SDS of height, weight and IGF-I or IGFBP-3 are presented in
Table 2. SDS of height and weight were significantly correlated to
IGF-I levels, in the 4-10 (r=0.472, p=0.0001 and r=0.443, p=0.0001
respectively) and 11-15 years age group (r=0.445, p=0.0001 and r=0.539,
p=0.0001 respectively). Simil 1000 ar significant correlations were
also obtained between SDS of height and weight with IGFBP-3, but only
amongst the younger children (r=0.494, p=0.0001 and r=0.489, p=0.0001
respectively). In the older age group, correlations were weaker though
still significant (IGFBP-3 vs. height SDS, r=0.296, p<0.01; IGFBP-3
vs. weight SDS, r=0.313, p<0.01).
Figure 3. Correlation between IGF-I and IGFBP-3
levels of normal children.
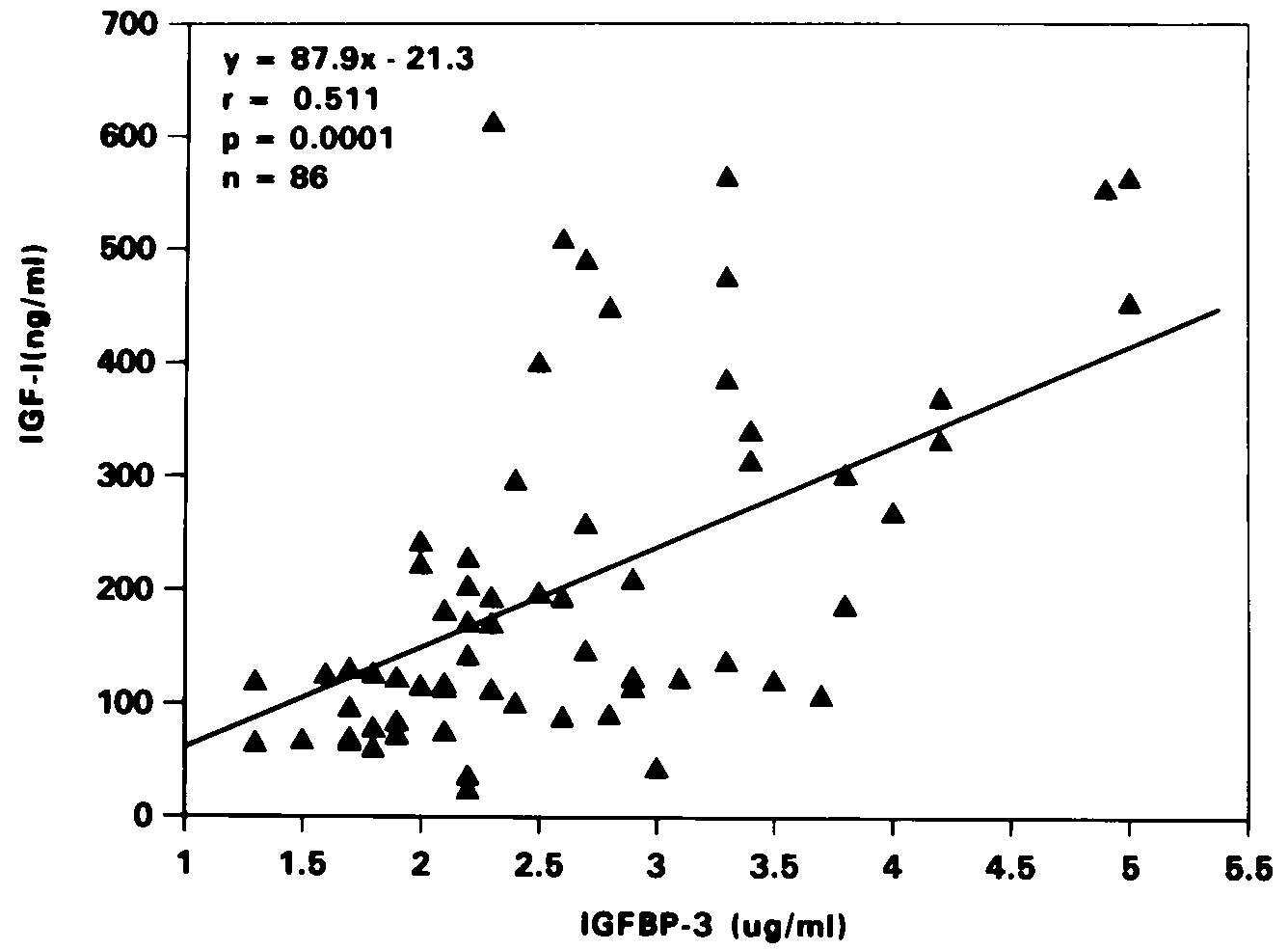
Figure 4. Correlation between IGF-I and IGFBP-3
levels of moderate and severely stunted malnourished children.
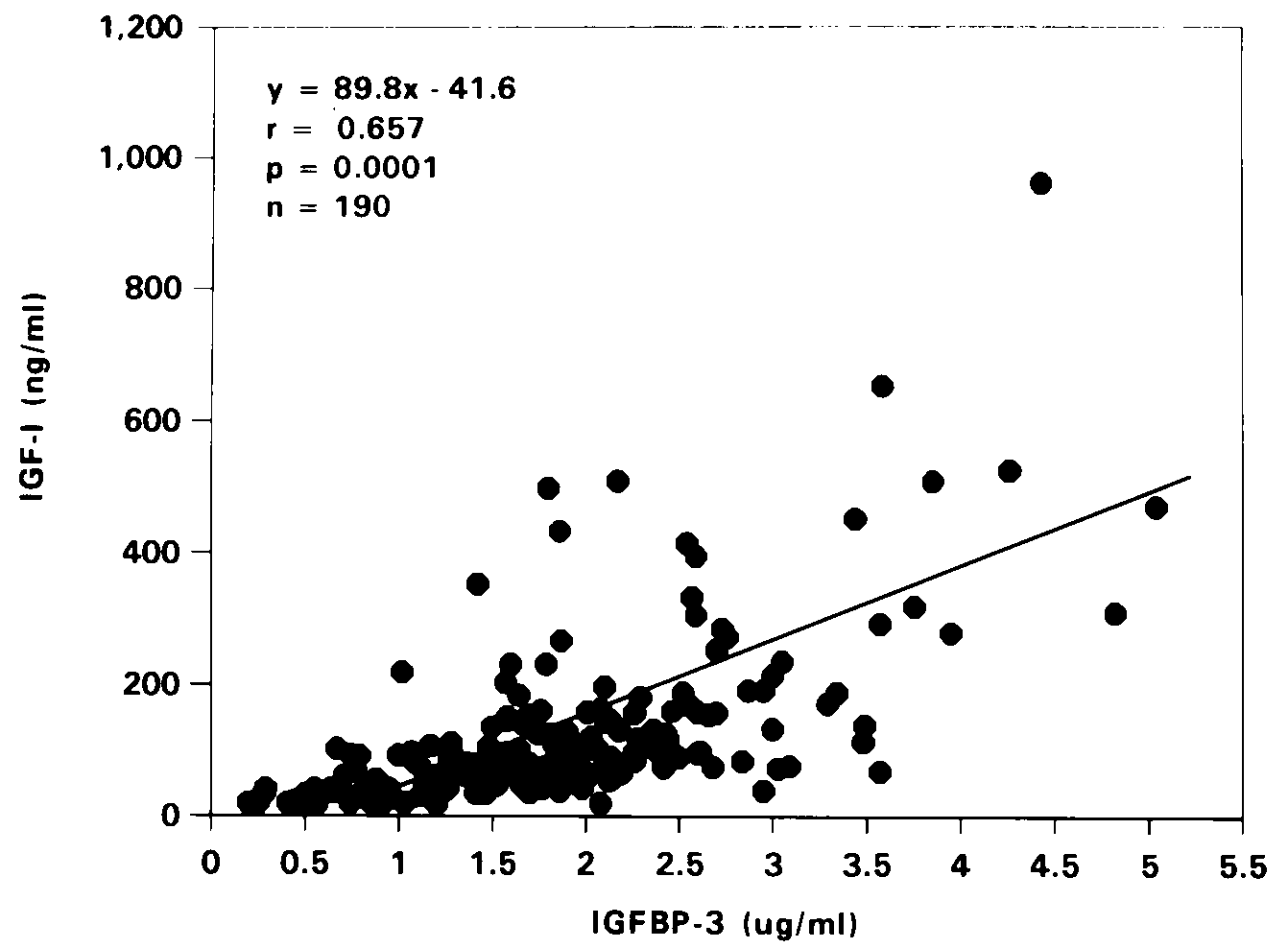
Table 2. Correlations between SDS of height,
weight and plasma IGF-I or IGFBP-3 levels in study subjects.
| |
|
4-10 years old
|
11-15 years old
|
| Constant |
Variable |
r
|
p
|
r
|
p
|
| Height SDS |
IGF-I |
0.472
|
0.0001
|
0.445
|
0.0001
|
| Weight SDS |
IGF-I |
0.443
|
0.0001
|
0.539
|
0.0001
|
| Height SDS |
IGFBP-3 |
0.494
|
0.0001
|
0.296
|
<0.01
|
| Weight SDS |
IGFBP-3 |
0.489
|
0.0001
|
0.313
|
<0.01
|
Discussion
In underprivileged populations, poor growth in children
is mostly caused by inadequate food intake and associated repeated
parasitic infections. Various anthropometric indices have been used
to assess growth status. These include, height for age which represents
linear growth and measures long-term growth faltering, weight for
height which reflects body proportion, and weight for age which is
a representation of both linear growth and body proportion7.
As shown in this study, childhood malnutrition has resulted in moderate
and severe growth retardation in a group of children. The low IGF-I
and IGFBP-3 levels in moderate and severely stunted children were
consistent with the results of previous studies where malnutrition
due to coeliac disease8, short-term fasting9
and anorexia nervosa10 caused significant reduction in
both IGF-I and IGFBP-3 levels. We have also reported a similar observation
in our earlier study11. Using body mass index (BMI) to
categorise our malnourished children, IGF-I of mildly (BMI=15-18 kg/m2)
and moderately (BMI <15 kg/m2) malnourished children
of age-group 4-10 years were found to be significantly lower than
those with normal BMI (>18 kg/m2). In this study, IGF-I
was found to be more sensitive than IGFBP-3 to the nutritional status.
All malnourished children had significantly lower IGF-I levels compared
to normal children. On the other hand, IGFBP-3 levels were significantly
lower than normal children only in the younger age group. This binding
protein normalised in the older children, and the difference was significant
only in the severely stunted children. The explanation for this observation
is possibly related to the pubertal increase in sex steroids and growth
hormone12-14 which in this case, resulted in normalisation
of IGFBP-3 but not IGF-I, which remained significantly low.
As reported by others15,16, IGF-I and IGFBP-3
levels of the normal children were significantly correlated. Malnutrition
has been known to cause derangement in the GH/IGF-I axis, resulting
in elevated GH and low IGF-I levels2,5,9,10. In this study,
however, IGF-I levels of the malnourished children were found to be
positively and highly significantly correlated to IGFBP-3 levels,
indicating that both IGF-I and IGFBP-3 were equally affected in mild
to moderate malnutrition. In addition, we have also observed that
in spite of the malnutrition and impaired growth, there was still
significant age-related increase in IGF-I and IGFBP-3 levels, similar
to that seen in the normal children.
A number of previous studies have shown that IGF-I
SDS correlated positively and significantly with height SDS of only
prepubertal, but not pubertal subjects16,17. In this study,
however, IGF-I levels of all children were found to correlate significantly
with the SDS of height and weight, while association between IGFBP-3
and SDS of height or weight was observed only amongst the older children.
Thus, our results showed that nutrition exerts a greater effect on
IGF-I compared to IGFBP-3, suggesting that this growth factor plays
a more important role in determining the linear growth of nutritionally
deprived children.
1000 Acknowledgments. This work was supported by a grant from the IRPA program no.
03-07-03104, from the Ministry of Science, Technology and Environment,
Malaysia. The authors are grateful to Ms Norhazwati and Mr Ghazali
for their technical assistance and also wish to thank the Director
of the Institute for Medical Research, for permission to publish this
study.
References
- Allen LH. The nutrition CRSP: what is marginal
malnutrition, and does it effect human function? Nutr Revs 1993;
51(9): 255-267.
- Maes M, Maiter D, Thissen J-P, Underwood LE, Ketelslegers
J-M. Contributions of growth hormone receptor and postreceptor defects
to growth hormone resistance in malnutrition. Trends Endocrinol
Metab 1991; 2: 92-97.
- Teale JD, Marks V. The measurements of insulin-like
growth factor l: clinical applications and significance. Ann Clin
Biochem 1986; 23: 413-424.
- Clemmons DR, Van Wyk JJ. Factors controlling blood
concentration of somatomedin-C. Clin Endocrinol Metab 1984; 13:113-143.
- Thissen JP, Triest S, Underwood LE, Maes M, Ketelslegers
JM. Divergent responses of serum insulin-like growth factor-l and
liver growth hormone (GH) receptors to exogenous GH in protein restricted
rats. Endocrinology 1990; 126: 908-913.
- Lachance PA. Recommended dietary allowances for
growth, development and performance. Asia Pacific J Clin Nutr 1995;
4(suppl 1):7-12.
- de Onis M, Monteiro C, Akre J, Clugston G. The
worldwide magnitude of protein-energy malnutrition: an overview
from the WHO global database on child growth. Bull World Health
Organization 1993; 71 (6):703-712.
- Hernandez M, Argente J, Navarro A, Caballo N, Barrios
V, Hervas F, Polanco I. Growth in malnutrition related to gastrointestinal
diseases: coeliac disease. Horm Res 1992; 38(suppl 1):79-84.
- Smith WJ, Underwood LE, Clemmons DR. Effects of
caloric or protein restriction on insulin-like growth factor-I (IGF-I)
and IGF-binding proteins in children and adults. J Clin Endocrinol
Metab 1995; 80: 443-449.
- Counts DR, Gwirtsman H, Carlsson LMS, Lesem M,
Cutler GB. The effect of anorexia nervosa and refeeding on growth
hormone-binding protein, the insulin-like growth factors (IGFs),
and the IGF-binding proteins. J Clin Endocrinol Metab 1992; 75:762-767.
- Wan Nazaimoon WM, Osman A, Ng ML, Tan TT, Wu LL,
Sakinah O, Khalid BAK. Insulin-like growth factor-l and fasting
growth-hormone levels in mild and moderately malnourished children.
Asia Pacific J Clin Nutr 1992; 1:207-210.
- Kerrigan JR, Rogol AD. The impact of gonadal steroid
hormone action on growth hormone secretion during childhood and
adolescence. Endocr Rev 1992; 13:281-298.
- Argente J, Barrios V, Pozo J, et al. Normative
data for insulin-like growth factors (IGFs), IGF-binding proteins,
and growth hormone-binding protein in a healthy Spanish pediatric
population: age- and sex-related changes. J Clin Endocrinol Metab
1993; 77: 1522-1528.
- Metzger DL, Kerrigen JR, Rogol AD. Gonadal steroid
hormone regulation of the somatotropic axis during puberty in humans.
Trends Endocrinol Metab 1994; 5:290-296.
- 8d8 Blum WF, Albertsson-Wikland K, Rosberg S,
Ranke MB. Serum levels of insulin-like growth factor I (IGF-I) and
IGF binding protein 3 reflect spontaneous growth hormone secretion.
J Clin Endocrinol Metab 1993; 76:1610-1616.
- Strasser-Vogel B, Blum WF, Past R, Kessler U, Hoeflich
A, Meiler B, Kiess. Insulin-like growth factor (IGF)-I and -II and
IGF-binding proteins-l, -2 and -3 in children and adolescents with
diabetes mellitus: correlation with metabolic control and height
attainment. J Clin Endocrinol Metab 1995; 80: 1207-1213.
- Dacou-Voutetakis C, Dracopoulou M, Georgopoulos
N, et al. Height, IGF 1, GH and HbA1 in children and adolescents
with insulin-dependent diabetes mellitus. 75th Annual Meeting of
The Endocrine Society, Las Vegas, NV, 1993 (Abstract 738c).
Effects of childhood malnutrition
on Insulin-like Growth Factor-l (IGF-I) and IGF-Binding Protein-3
levels: a Malaysian and New Zealand analysis
Wan Nazaimoon WM, Rahmah R, Osman A,
Khalid BAK, Livesey J
Asia Pacific Journal of Clinical
Nutrition (1997) Volume 6, Number 4: 273-276

Copyright © 1997 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
to the top
0