1000
Asia Pacific J Clin Nutr (1997) 6(3): 219-223
Asia Pacific J Clin Nutr (1997)
6(3): 219-223

Amino
acid levels following beef protein and amino acid supplement in male
subjects
Anthony M Uhe MSc, Kerin O’Dea
PhD and Greg R Collier PhD
School of Nutrition and Health, Deakin
University, Geelong, Australia
In the present study the plasma amino acid response
of six lean subjects to a protein meal and a commercial amino acid
supplement were compared. The amino acid supplement studied was
formulated and marketed to be taken after exercise and at other
times with the aim of increasing protein synthesis and/or decreasing
protein degradation and to lower the ratio of tryptophan to the
other large neutral amino acids (LNAA); tyrosine, valine, leucine,
isoleucine, phenylalanine and methionine (trp/LNAA), to reduce fatigue.
The amino acid supplement administered at the dose recommended by
the manufacturer (4 g) was able to bring about a rapid but short-lived
(15-30 min) increase in plasma amino acid concentrations and to
produce a similarly brief decrease in the trp/LNAA and tyr/LNAA
ratios and therefore achieved these aims with respect to amino acid
levels even if only briefly. The changes in trp/LNAA and tyr/LNAA
ratios after the supplement were of the same order as those produced
after the much larger (50 g) protein meal but of shorter duration.
However the relatively small insulin response after the amino acid
supplement points to a lower level of amino acid uptake by muscle
and other tissues for protein synthesis compared to that produced
by the beef meal.
Key words: Amino acids, beef, supplements,
insulin, LNAA (large neutral amino acids)
Introduction
In view of the current popularity of amino acid supplements,
particularly with athletes, we compared a commercially available amino
acid supplement with a muscle protein source, lean beef to determine
the relative effectiveness of these two sources of amino acids in
modulating the plasma amino acid profile. Amino acid supplements are
marketed with many claims including enhancement of sporting performance,
increasing protein synthesis, decreasing protein degradation, accelerating
tissue repair and altering neurotransmitter levels affecting fatigue
and mood. It has even been proposed that effects such as weight loss
and increased fatty acid oxidation can be produced by amino acid supplements1-5.
There is a large range of different amino acid supplements available
(from single amino acids to complex mixtures), but a paucity of information
in the literature on their effects, benefits and hazards in humans.
Most research on the administration of amino acids to humans has been
in the area of parenteral and enteral nutrition and not 1000 in supplementation
of the diets of healthy, active individuals or elite athletes.
One aspect of amino acid supplementation potentially
of interest, is whether they effect the levels of the precursors of
neurotransmitters. Monoamine neurotransmitters such as serotonin (5-hydroxytryptamine)
and the catecholamines (noradrenaline and dopamine) have been implicated
in the regulation of fatigue, food intake, mood, pain sensitivity
and sedative effects1,4,6,7. The level of serotonin and
catecholamines are influenced by the level of their precursors tryptophan
(trp) and tyrosine (tyr) in the brain which in turn depend on the
competition between these amino acids and the other large neutral
amino acids (LNAA, tyrosine, valine, leucine, isoleucine, phenylalanine
and methionine6 for transport across the blood brain barrier.
Amino acid supplements have the potential to alter
or modulate all of these effects. However, the plasma amino acid profile
following amino acid supplements has not been investigated. In the
current study we compared the amino acid profile of the test meal
with the plasma amino acid profiles, glucose and insulin levels in
six lean male subjects in response to a meal of lean beef containing
50g of protein and 4g of the amino acid supplement.
Experimental
Subjects
Six lean male subjects were recruited: age 19.5 ±
1.2 years (mean ± SEM), (range 17 - 25), and mean body mass index
BMI 21.7 ± 0.7 kg/m2 (range 19.4 - 23.4). Only male subjects
were included in this study because of reports of changes in trp/LNAA
ratios during the menstrual cycle7. Ethics approval was
obtained from the Human Ethics Committee at Deakin University and
all subjects gave their informed consent.
Protocol
Subjects consumed two "meals" in random
order after an overnight fast: 230g of grilled lean topside steak,
equivalent to 50g of protein and 6g of fat, and 4g of the amino acid
supplement "Kuan the Creative" (Musashi Pty Ltd Mulgrave
Victoria Australia). The dose was that recommended by the manufacturer:
a 4g dose twice a day or immediately after sport/exercise. Both the
amino acid supplement and the beef meal were taken with a glass of
water (200ml). Subjects were requested to chew their beef meal thoroughly
and complete the meal within 15 minutes, the amino acid supplement
was consumed in the form of a powder. To control activity the subjects
remained seated during the course of the study.
Blood samples were collected from a soft indwelling
catheter inserted into an antecubital vein 15 minutes before and immediately
before commencing the meal and then at 15, 30, 45, 60, 90, 120, 150
and 180 minutes after the commencement of eating. Blood samples were
collected into 5 ml fluoride heparin tubes, placed on ice and the
plasma separated by centrifugation within 30 minutes of the last sample.
Samples were then stored frozen either at -20° C for glucose and insulin or –70° C for amino acid measurements.
Analysis
Amino acids were measured by an automated HPLC method
with ophthaldialdehyde pre-column derivatisation and separation on
a reversed phase column. Samples were deproteinised with acetonitrile
prior to analysis and homoserine was used as the internal standard,
the coefficient of variation for the assay was 5.2% for plasma samples8.
The amino acid supplement (3 1000 00mg) was mixed with water and acidified
with HCl to ensure that the amino acids had dissolved and made up
to a total volume of 100ml, a sample of this solution was then analysed
as for the plasma samples. The amino acid composition of the beef
was determined by the same method after lipid extraction and hydrolysis
with HCl and 2-mercaptoethanol9. The nonprotein amino acid
content of the beef protein was also measured in tissue homogenate
to assess the contribution of nonprotein amino acids to dietary levels.
A sample of beef was homogenised with a Ystral x10/20 tissue Homogeniser
(Ystral GMBH Ballrechten-Dottingen) and 100µg sample of homogenate
was analysed after deproteinisation as per the amino acid analysis
method. The amino acid composition reported are the sum of the acid
hydrolysis and tissue homogenate and are in the form of the free base.
Glucose was measured in plasma by glucose oxidase assay (Peridochrom
Glucose Boehringer Mannheim GMBH Diagnostica) on a centrifugal analyser
(Centrifichem 550 Baker Instruments). Insulin was measured by radioimmunoassay,
double antibody solid phase technique (Phadeseph Insulin RIA, Pharmacia
Diagnostics AB Uppsala, Sweden). The fat content of the beef was determined
by CEM rapid automated moisture and fat analyser (CEM Corp Indiana
Trail NC, USA)10 and was 1.6% wet weight.
Statistical
analysis
Analysis of variance (two-way ANOVA with. randomised
block design) was used to determine the significance of any differences
between the treatment groups and individuals with respect to glucose,
insulin, amino acid profiles, trp/LNAA ratio and tyr/LNAA ratio profiles
over time. Computer packages (Open Access 3 SPI) and Minitab were
used for statistical analysis. Regression analysis was also performed
where appropriate. P values < 0.05 were considered significant,
all values are expressed as mean ± SEM.
Results
Amino
acid composition of meals
Amino acid composition of beef from protein hydrolysis
and homogenate was also analysed to determine the level of both protein
and non-protein amino acids. The composition of the amino acid mixture
was also analysed (Table 1). As would be expected the amino acid content
of the beef meal was greater than the amino acid supplement. The amino
acid supplement did not contain all amino acids found in protein.
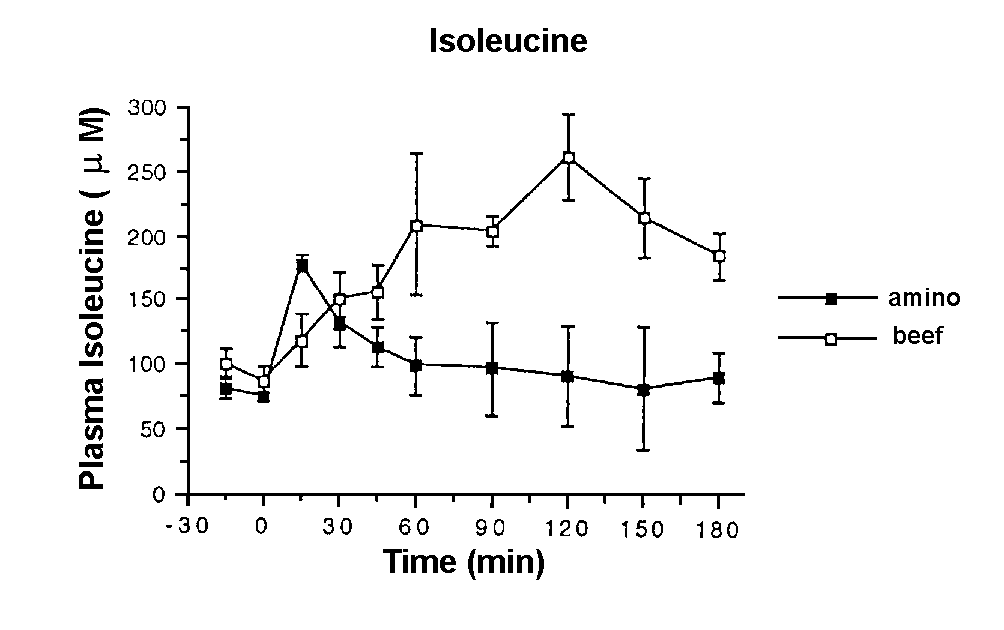
Figure 1. Plasma isoleucine levels in response
to a meal of 50g of protein in the form of lean beef topside (o ) and 4g of the Musashi amino acid supplement (n ) in 6 lean male subjects.
Plasma
Amino Acids
Amino acid profiles were measured for 20 plasma amino
acids. The profiles for the two meals were different with isoleucine
being a representative of amino acids present in the amino acid supplement
(Figure 1). Amino acid levels took significantly longer to reach peak
levels after the beef meal (111 ± 8 minutes) than after the amino
acid supplement (19 ± 2 minutes) (p < 0.01), this effect was consistent
across all the amino acids measured.
Table 1. Amino acid content of each meal (g).
| Amino acid (in the
form of free base) |
Beef
50g protein
|
Amino acid supplement
4g supplement
|
| Aspartic acid (asp)
& Asparagine (asn) |
7.9
|
n
|
| Glutamic acid (glu)
& Glutamine (gln) |
10.2
|
n
|
| Serine (ser) |
4.2
|
n
|
| Histidine (his) |
1.1
|
0.26
|
| Glycine (gly) |
4 3
|
0.33
|
| Threonine (thr) |
4.0
|
0.25
|
| Arginine (arg) |
3.6
|
0.13
|
| Taurine (tau) |
0.23
|
n
|
| Alanine (ala) |
4.6
|
n
|
| Tyrosine (tyr) |
1.9
|
0.13
|
| Tryptophan (trp) |
0.07
|
n
|
| Methionine
(met) |
0.75
|
0.04
|
| Valine (val) |
3.4
|
0.25
|
| Phenylalaninie (phe) |
1.7
|
0.28
|
| Isoleucine (ile) |
3.0
|
0.5
|
| Leucine (leu) |
3.9
|
0.9
|
| Lysine (lys) |
3.4
|
0.19
|
n - not present in supplement.
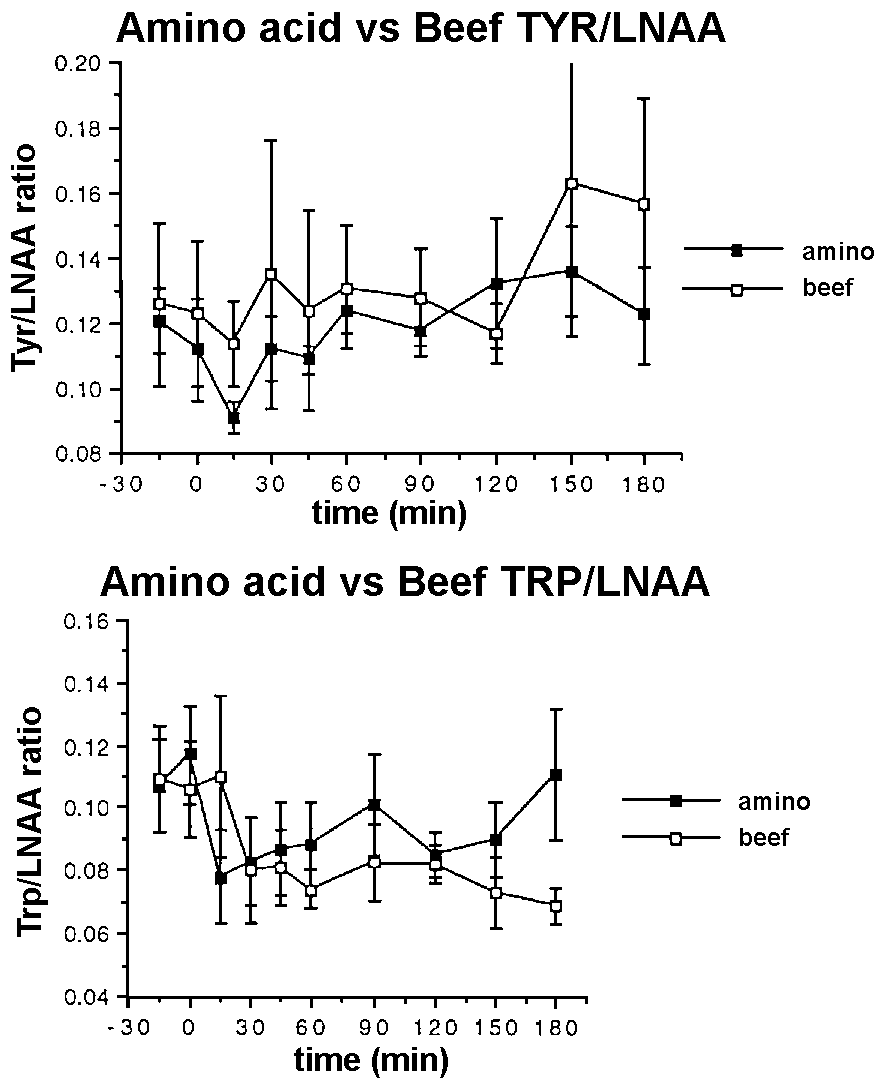
Tyr/LNAA
and trp/LNAA ratios
The tyr/LNAA ratios reached a minimum at 15 minutes
after both meals. The tyr/LNAA ratio being significantly lower at
15 minutes after the amino acid supplements (p < 0.05) than the
beef meal (Figure 2a). Trp/LNAA ratio declined significantly from
baseline after both the meals (p < 0.05) with the minimum being
reached earlier after the amino acid meal than the beef meal (Figure
2b). However the ratio returned to normal for the amino acid supplement
while the ratio after the beef meal was still depressed after 180
minutes although the differences between the two treatments with respect
to the trp/LNAA ratio failed to reach the level of significance.
Figure 2. Plasma tyr/LNAA (a) and trp/LNAA
ratio (b) in response to a meal of 50 g of protein in the form of
lean beef topside (o ) and 4g of the Musashi amino acid supplement
(n ) in 6 lean male subjects.
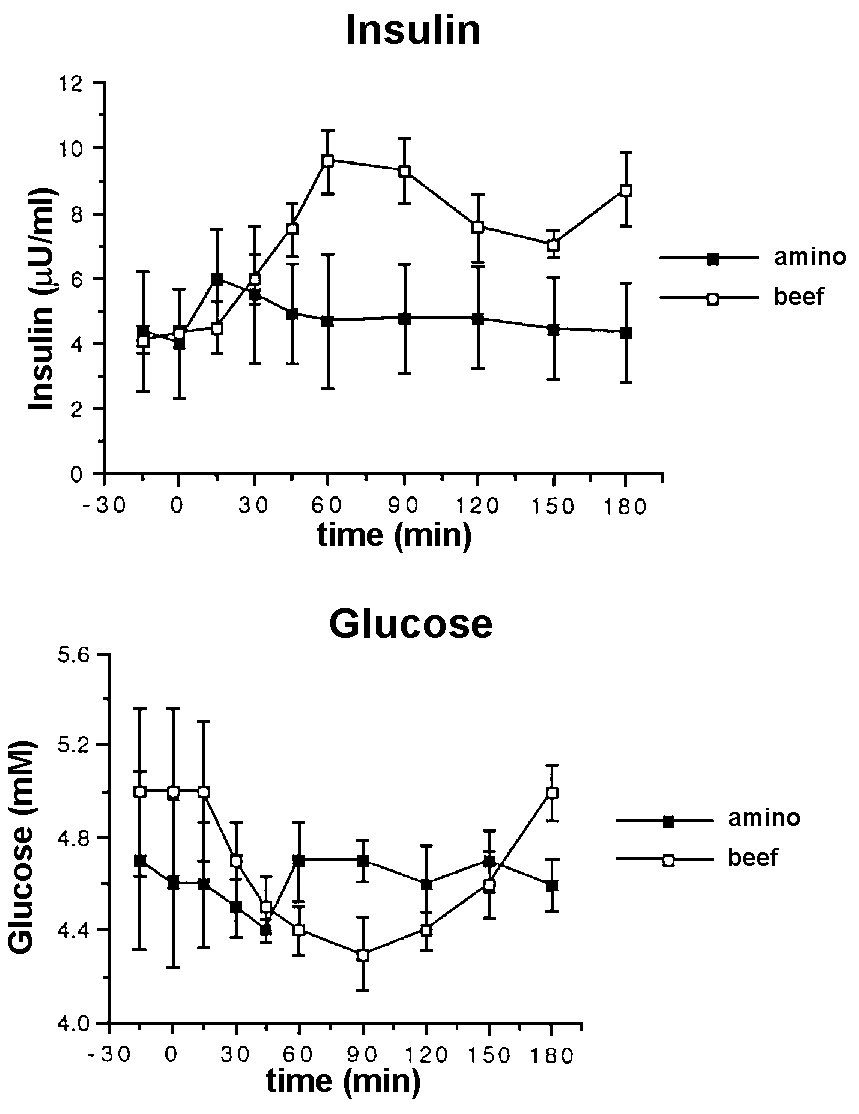
Insulin
and Glucose
Insulin levels increased significantly more after
the beef meal compared with the amino acid supplement (Figure 3a)
(p < 0.05). The insulin values reached a peak at 15 and 60 minutes
for the amino acid supplement and beef respectively. Correspondingly,
glucose levels declined significantly after both meals with minimum
values at 45 and 90 minutes for amino acid supplement and beef respectively
(Figure 3b) (p < 0.05 and p < 0.01 respectively).
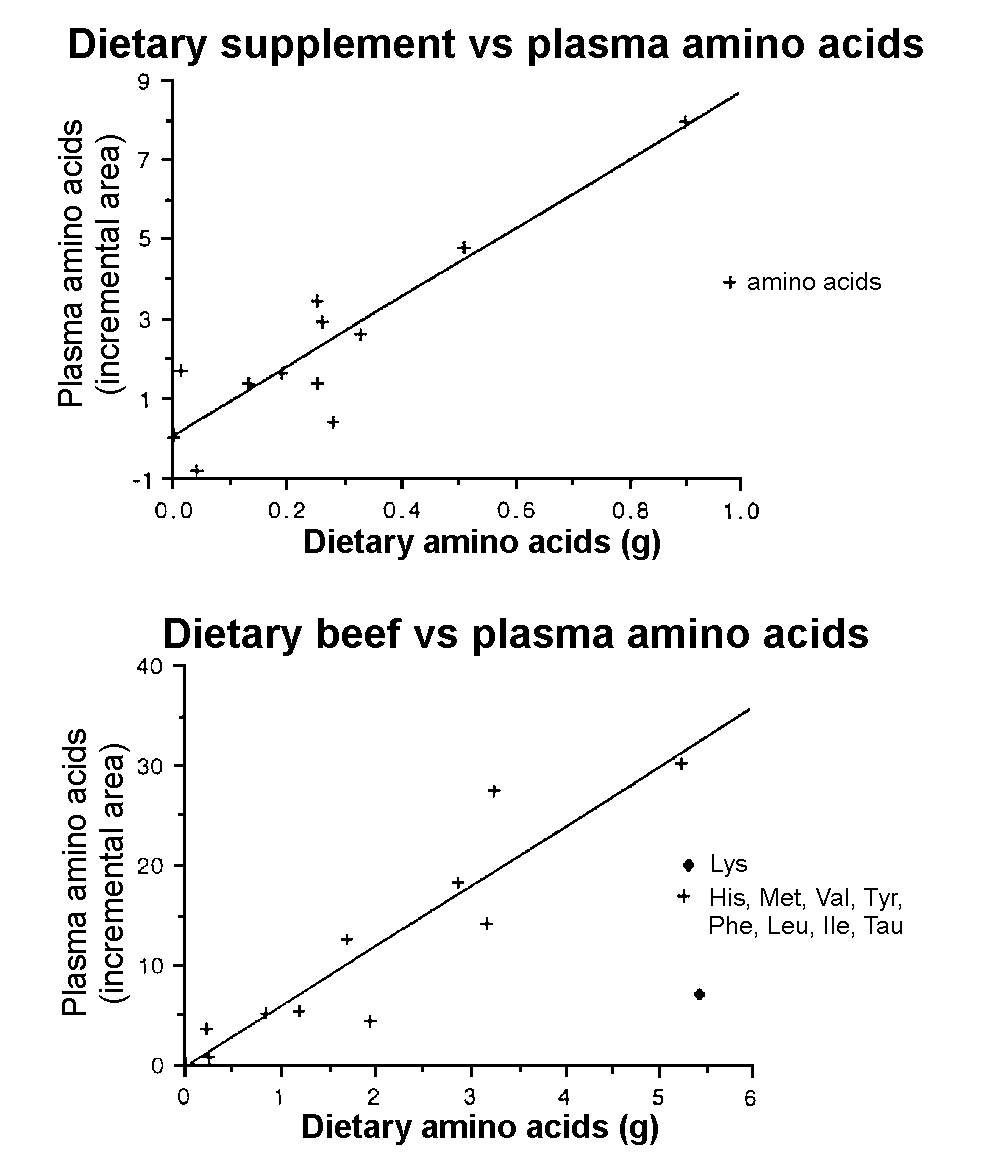
Correlation
between dietary and plasma amino acid levels
The amino acid composition of meals was plotted against
the increase in plasma amino acid levels expressed as total incremental
area (the incremental area being the 1000 area above baseline under
the curve of the amino acid response) (Figure 4). Linear regression
showed good correlation with essential amino acids except for lysine
after the beef meal (4a) and good correlations with all amino acids
after the amino acid supplement (4b) (r = 0.927 and 0.930 for the
beef and amino acid supplement respectively).
Discussion
The beef meal was larger in volume and contained more
amino acids, in the form of protein (50g) than the supplement which
contained free amino acids (4g). However the aim of this study was
to give the supplement at the recommended dose and to compare this
with the response to a standard protein meal. This particular amino
acid supplement is formulated (according to the manufacturer) to be
taken after exercise and at other times with the aim of increasing
protein synthesis and/or decreasing protein degradation ("allowing
a man to rebuild his body to its ideal state" according to the
advertising material) and to lower the trp/LNAA ratio, purportedly
to reduce fatigue. From our observations the amino acid supplement
was able to bring about a rapid but short-lived increase in plasma
amino acid concentrations and to produce a decrease in the trp/LNAA
and tyr/LNAA ratios.
Figure 3. Plasma insulin (a) and plasma glucose
(b) in response to a meal of 50 g of protein in the form of lean beef
topside (o ) and 4g of the Musashi amino acid supplement (n ) in 6 lean male subjects.
The amino acid supplement resulted in a rapid increase
(15 minutes) and subsequent decline in plasma amino acids indicating
that the free amino acids found in the amino acid supplement are absorbed
quickly into the bloodstream and cleared quickly (within 60 minutes
of ingestion). However after the beef meal plasma amino acids did
not peak until much later (120-150 minutes) reflecting the time that
would be needed to digest the protein in the beef and absorb the resulting
amino acids and peptides. Plasma levels of most amino acids were elevated
significantly above baseline from 30 minutes after ingestion of the
beef meal and were maintained for at least three hours compared to
the more transient increase after the amino acid supplement.
The tyr/LNAA ratio reached a minimum at 15 minutes
after both meals with the decline being significantly greater after
the amino acid supplements than the beef meal. Trp/LNAA ratio declined
significantly after both the meals with the decline after the amino
acid meal being faster than that for the beef meal. However, the ratio
returned to normal levels while the ratio after the beef meal was
still depressed after 180 minutes. The differences between the trp/LNAA
ratio for the beef and the amino acid supplement were not statistically
significant. The decline in the trp/LNAA and tyr/LNAA ratio is due
to relatively lower levels of trp and tyr in muscle protein and absence
of trp and low levels of tyr in the amino acid supplement compared
to the levels of the other LNAA (Table 1). It is notable that a small
amount of amino acid supplement (4g) was able to produce effects (at
least briefly) on these amino acid ratios of the same order or greater
than a large protein meal (50g), but of much shorter duration. The
physiological significance of these changes in plasma amino acids
is unknown. The putative effects of these changes on mood and fatigue
were not measured in this study.
Figure 4. Correlation between dietary amino
acid levels (g) and plasma amino acid levels (incremental area) in
response to meal of 50g of protein in the form of (a) lean beef topside
( + ) (A 1000 mino acids: His, Met, Val, Leu, Ile, Tyr, Tau, Phe),
r = 0.914, (u ) Lys and (b) 4 g of the Musashi amino
acid supplement ( + ) (Amino acids: His, Gly, Hr, Rag, Tyr, Met, Val,
Phe, Ile, Leu, Lys), r = 0.930 in six lean male subjects.
Several recent studies have looked at the effect of
exercise on amino acid concentration and metabolism1,11-13.
Amino acid supplements have the potential to alter or modulate metabolism
after exercise, by changes in trp/LNAA and tyr/LNAA ratios and in
the levels of other important amino acids such as gluconeogenic precursors
and branched chain amino acids. However, more work is needed on the
short term nature of the changes in amino acid levels compared with
the longer term changes seen after the beef meal before the potential
effects flowing from them can be characterised.
The insulin level increased significantly more after
the beef meal than the amino acid supplement. However, the increase,
while large in percentage terms, was only small compared with that
after glucose or a mixed meal. The increased insulin response to the
beef meal is attributable to the greater quantity of amino acids released
after hydrolysis compared with the supplement (50g of protein vs 4g
free amino acids) via amino acid stimulation of insulin release14-16.
Insulin levels peaked at 15 minutes for the amino acid supplement
in agreement with the peak amino acid levels. However the insulin
peaked earlier than the amino acid levels after the beef meal (60
vs 120 minutes). The greater insulin response to the beef meal compared
with the amino acid supplement would be expected to coincide with
increased amino acid uptake into muscle and other tissues and protein
synthesis. This would suggest that the anabolic effects of the beef
meal would be greater than that produced by the amino acid supplement.
Glucose levels declined by a small but significant amount after both
meals with minimum values at 45 and 90 minutes for amino acid supplement
and beef respectively probably reflecting the earlier insulin response
to the amino acid supplement compared to the beef meal (15 vs 60 minutes).
These data are similar to the results reported by Schmid et al16
after a tenderloin pork meal and intravenous amino acid infusions.
There was good correlation between plasma amino acid
and the amino acids found in the supplement; the correlation was similarly
strong for all essential amino acids (EAA) except for lysine. Relative
plasma concentrations of lysine increased proportionately less than
the other EAA. Of the non-essential amino acids only taurine increased
to the same extent as the EAA. Plasma alanine, arginine, serine and
glycine increased to a lesser extent than the EAA. Aspartate/asparagine
and glutamate/ glutamine showed very little increase in plasma levels
compared to dietary levels probably due to splanchnic and hepatic
uptake3. This is in accordance with the findings of other
workers who have found similar correlations between dietary and plasma
amino acid in both humans15,17,18 and the rat19.
It would be possible on the basis of this sort of correlation to predict
with some degree of accuracy the likely plasma response to amino acid
supplements and to a lesser extent dietary protein in the absence
of carbohydrate induced insulin effects.
Conclusion
The particular amino acid supplement studied is formulated
to be taken after exercise and at other times with the aim of increasing
protein synthesis and or decreasing protein degradation and to lower
the trp/LNAA ratio. From our observations the amino acid supplement
was able to bring about rapid but short-lived incre 1000 ase in plasma
amino acid concentrations and to produce a decrease in the trp/LNAA
and tyr/LNAA ratios. However the physiological significance of these
changes in plasma amino acids is unknown. The relatively small insulin
response after the amino acid supplement points to a lower level of
amino acid uptake by muscle and other tissues for protein synthesis
compared to that produced by the beef meal.
References
- Blomstrand E, Celsing F, Newsholme EA. Changes
in plasma concentrations of aromatic and branched-chain amino acids
during sustained exercise in man and their possible role in fatigue.
Acta Phisiol Scand 1988; 133:115-121.
- Cerretelli P, Marconi C. L-carnitine supplementation
in humans. The effects on physical performance. Int J Sports Med
1990; 11:1-12.
- Christensen HN. Role of amino acid transport and
countertransport in nutrition and metabolism. Physiol Rev 1990;
70:43-71.
- Lopez-Ibor JJ. The involvement of Serotonin in
Psychiatric disorders and behaviour. Brit J Psych 1988; 153:26-39.
- May ME, Buse MG. Effects of branch-chain amino
acids on protein turnover. Diabetes/Metabolism Rev 1989; 5:227-245.
- Anderson GH. Metabolic regulation of food intake.
In: Shils ME, Young VR, eds. Modern Health and Disease 7th edition.
Philadelphia: Lea and Febiger 1989, 557-569.
- Anderson GH, Li ETS. Protein and amino acids in
the regulation of quantitative and qualitative aspects of food intake.
Int J Obesity 1987, 11(suppl 3):97-101.
- Uhe AM, Collier GR, McLennan EA, Tucker DJ, O’Dea
K. The quantitation of tryptophan and other plasma amino acids by
automated precolumn derivatization high performance liquid chromatography:
Improved sample preparation. J Chromatogr 1991; 564:81-91.
- Ng LT, Pascaud A, Pascaud M. Hydrochloric acid
hydrolysis of proteins and determination of tryptophan by reversed-phase
high performance liquid chromatography. Anal Biochem 1987; 167:47-52.
- Mann N, Sinclair A, Watson M, O’Dea K, Evaluation
of rapid fat determination in meats using the CEM automated analyser.
Food Australia 1991; 43:67-69.
- Conlay LA, Wurtman RJ, Lopz G-Coviella I, Blusztajn
JK, Vacanti CA, Logue M, During M, Caballero B, Maher TJ, Evoiuk
G. Effects of running the Boston marathon on concentrations of large
neutral amino acids. J Neural Transm 1989; 76:65-71.
- Devlin JT, Brodsky I, Scrimgeour A, Fuller S, Bier
M. Amino acid metabolism after intense exercise. Am J Phisiol 1990;
258:E249-E255.
- Einsparh KJ, Tharp G. Influence of endurance training
on plasma amino acid concentrations in humans at rest and after
intense exercise. Int J Sports Med 1989; 10:233-236.
- Beylot M, Chambrier C, Moneger A, Cohen R. Effect
of small variations in insulin and glucagon levels on plasma amino
acids concentrations. Diabete & Metabolisme 1989; 15:38-44.
- Nuttall FQ. Effect of protein ingestion on the
glucose and insulin response to a standardized oral glucose load.
Diabetes Care 1984; 7:465-470.
- Schmid R, Schusdziarra V, Schulte-Frohlinde E,
Maier V, Classen M. Role of amino acids in stimulation of postpran
7b9 dial insulin, glucagon and pancreatic polypeptide in humans.
Pancreas 1989; 4:305-314.
- Ashley DV, Barclay DV, Chauffard FA, Moennoz D,
Leathwood PD. Plasma amino acid response in humans of differing
nutritional composition. Am J Clin Nutr 1982; 36:143-153.
- Fernstrom JD, Wurtman RJ, Hammarstrom-Wiklund B,
Rand WM, Munro N, Davidson CS. Diurnal variations in plasma concentrations
of tryptophan, tyrosine, and other neutral amino acids: effect of
dietary protein intake. Am J Clin Nutr 1979; 32:1912-1922.
- Johnson D, Anderson GH. Prediction of plasma amino
acid concentration from diet amino acid concentration. Am J Physiol
1982; 243:R99-R103.
Amino acid levels following beef
protein and amino acid supplement in male subjects
Anthony M Uhe, Kerin O’Dea and Greg R Collier
Asia Pacific Journal of Clinical Nutrition (1997) Volume 6, Number
3: 219-223

Copyright © 1997 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
to the top
0