Asia Pacific J Clin
Nutr (1997) 6(1): 26-30

Dietary
oleic and palmitic acid exert similar effects on plasma lipids and
lipoprotein metabolism in hamsters fed purified diets with low cholesterol
but different quantities of fat
Pramod Khosla PhD, Andrzej Pronczuk
DSc, Tahar Hajri PhD and
KC Hayes DVM, PhD
Foster Biomedical Research Laboratory,
Brandeis University, Waltham, MA, USA
The current study was designed to determine whether
the transition from a low-fat (20% en) to a high-fat (40% en) diet
through incremental increases in specific fatty acids (16:0 or 18:1)
would exert a differential effect on plasma lipids and lipoprotein
metabolism. Male Golden Syrian hamsters were fed purified diets
in which the dietary fat (fatty acids) content was varied by blending
different dietary oils. Animals were fed one of 5 purified diets:
a low-fat control diet with 20% en fat; two diets with 30%en from
fat; and two diets with 40% en from fat. In each case the extra
fat was supplied either by oleic acid or palmitic acid. Dietary
myristic (0 - 0.50% en) and linoleic acid (5.2 -5.9% en) were relatively
constant across all diets, which contained a low level of cholesterol
(~40 mg/1000 kcal). Diets were formulated so that protein, vitamins
and minerals were constant per calorie. All animals were fed a fixed
amount of calories for 6-8 week periods. Plasma lipids (n=15-20
per group) and lipoprotein cholesterol concentrations were determined
following sequential ultracentrifugation, from individual animals
(n=5-6 per group). Increasing dietary fat from 20% en to 30% en
to 40% en, by increasing oleic acid (6.9% en to 16.5% en to 24.8%
en respectively), did not affect total cholesterol (TC), triglyceride
(TG) or lipoprotein cholesterol concentrations. Similarly, increasing
dietary fat from 20% en to 30% en to 40% en, by increasing palmitic
acid (6.6% en to 12.9% en to 21 % en) had no affect on plasma lipids
or lipoprotein cholesterol. The similarity in plasma and lipoprotein
cholesterol levels was further confirmed by kinetic studies (at
8 weeks) in which animals were injected simultaneously with either
radiolabeled native LDL and methylated LDL or radiolabeled native
LDL and HDL. Consistent with the similarities in circulating LDL-C
concentrations, there was no difference in the clearance (ie fractional
catabolic rates and half-lives) of LDL by either receptor-mediated
or receptor-independent pathways. Similarly, in agreement with the
similar HDL-C concentrations, no difference was observed in HDL
fractional catabolic rates. Thus, if dietary myristic acid is low
and linoleic acid is adequate and constant, dietary 16:0 and 18:1
can be readily interchanged, across a wide range of energies without
compromising the plasma lipid profile in normocholesterolaemic animals
consuming low-cholesterol diets. Whether this 16:0/18:1 equivalence
is dependent on the relatively low levels of dietary cholesterol
and/or adequate amounts of linoleic acid (~5 to 6% en) remains to
be established.
Key words: Dietary fat, oleic acid,
palmitic acid, lipoprotein, LDL receptor activity
Introduction
Current dietary guidelines advocate reductions
in total fat consumption, saturated fatty acid and dietary cholesterol
intake, as a primary means for lowering low density lipoprotein cholesterol
concentrations (LDL-C), and thereby reducing subsequent risk from
coronary heart disease (CHD). While there is much data on the effects
of specific fatty acids on plasma lipids, the role of these individual
fatty acids within the context of the total fat load is relatively
unexplored. Most studies which have assessed fat quantity in modulating
plasma lipids, have utilised, at least in experimental animals, the
same basic fat fed at different levels in the diet1,2.
However, a distinct disadvantage in these types of studies is that
while total fat quantity and specific fatty acid content changes,
the relative mix or proportion of the various fatty acids remains
unaltered. Thus feeding a specific fat at 40% en and subsequently
at 30% en, results in decreased consumption of all the fatty acids
present in the parent fat, but the relative mix of fatty acids in
the parent fat is maintained at both fat loads. Hence the results
maintained may reflect decreased total fat consumption or decreases
in one or more fatty acids.
The current study was undertaken to explore what effect
the mix of fatty acids has when fat quantity alters. Previously,
using cholesterol-free purified diets fed to non human primates, or
gerbils and hamsters, the results of several studies from this laboratory
have revealed that under these conditions, myristic acid (14:0) and
linoleic acid (18:2) are key determinants of plasma lipids3-11.
When LDL receptors are down-regulated (either by feeding excessive
dietary cholesterol or for genetic reasons), palmitic acid (16:0)
becomes a major determinant of the resulting lipid response. These
studies have suggested that if the levels of 14:0 and 18:2 are controlled
in situations in which LDL receptor activity is not compromised, 16:0
and oleic acid (18:1) can be readily interchanged without impacting
plasma lipids. The results of recent human studies are in support
of this tenet12-15, but only when total fat intake is ~30%
en. This interchange does not appear, based on regression analyses9,10,
to depend on the total fat load. However the above mentioned studies
from this laboratory were carried out at only one fat level (30% en
in non-human primates and 40% en in hamsters and gerbils). Thus to
test the applicability of these findings to situations of multiple
fat levels (eg 20% en, 30% en and 40% en) the current study was conducted
in hamsters. The hypothesis tested was that using low levels of dietary
cholesterol, fat quantity could be altered - specifically by manipulating
16:0 and 18:1, without affecting plasma lipids or lipoprotein metabolism
provided 14:0 and 18:2 levels were unchanged. To test this objective,
hamsters were fed different purified diets in which dietary fat quantity
was varied (from 20% en ->30% en ->40% en) by blending different
dietary oils such that the increase in fat reflected increases in
either 16:0 or 18:1. Most importantly, the blends were prepared such
that regardless of the fat quantity, 14:0 levels (0-0.5% en) and 18:2
levels (5.2-5.9% en) were essentially constant across diets.
Materials
and Methods
Animals,
diets and study design. 101 male hamsters
(Lakeview strain: age ~6 months) were obtained from Charles River
Laboratories. They were housed 3-4 animals per cage and fed laboratory
chow (ad libitum) for 5 weeks. At this time, fasting plasma
cholesterol and triglyceride concentrations were determined in a random
subset of 33 animals. All animals were then switched to a cholesterol-free
low-fat purified diet (run-in diet) with 20% en from fat, and provided
with a fixed amount of diet per day (~52 kcal/animal/ day). After
2 weeks of feeding this diet, fasting plasma cholesterol and triglyceride
concentrations were determined in another random subset of 33 animals.
Animals were then randomly divided into 5 groups and each group was
assigned to one of 5 different purified diets (Table 1).
Table 1. Composition of hamster purified diets
(g/100 g)a
| Ingredient |
Diet lb
|
Diet 2
|
Diet 3
|
Diet 4
|
Diet 5
|
| Casein |
22.2
|
23.7
|
25.35
|
23.7
|
25.35
|
| Cornstarch |
34.75
|
25.9
|
16.15
|
25.9
|
16.15
|
| Glucose |
13.3
|
14.2
|
15.2
|
14.2
|
15.2
|
| Cellulose |
15.0
|
16.0
|
17.15
|
16.0
|
17.15
|
| Fat: (Total) |
8.25
|
13.25
|
18.75
|
13.25
|
18.75
|
| Palm Oil |
5.25
|
3.6
|
3.75
|
8.68
|
1.88
|
| Olive Oil-1 (7% 18:2) |
-
|
-
|
3.75
|
-
|
-
|
| Olive Oil-2 (15% 18:2) |
0.9
|
8.7
|
13.13
|
-
|
-
|
| Palm Stearin |
-
|
-
|
-
|
2.65
|
14.53
|
| Safflower Oil |
2.1
|
0.95
|
-
|
1.93
|
2.35
|
| Mineral mixc |
5.0
|
5.34
|
5.71
|
5.34
|
5.71
|
| Vitamin mixd |
1.2
|
1.28
|
1.37
|
1.28
|
1.37
|
| Choline Cl2 |
0.3
|
0.32
|
0.34
|
0.32
|
0.34
|
| Cholesterole |
0.015
|
0.016
|
0.017
|
0.016
|
0.017
|
(a) Diets were prepared in 2 kg batches by adding
120g of cornstarch to 1600mL water to make a gel. Then the remainder
of the diet was mixed in as the gel set. (b) Diet codes;
1 - low fat control diet; 2 - medium fat oleic acid-rich diet; 3 -
high fat oleic acid-rich diet; 4 - medium fat palmitic acid-rich diet
and 5 - high fat palmitic acid-rich diet. (c) Ausman-Hayes
mineral mix. (d) Hayes-Cathcart vitamin mix. (e) cholesterol
was dissolved in the hot oils prior to mixing with the remaining dietary
ingredients.
The control diet (Diet 1) was relatively low in fat
(20% en). Diet 2 was a medium-fat oleic acid enriched diet with 30%
en fat. Diet 3 was a high-fat oleic acid-enriched diet with 40% en
fat. Diet 4 was a medium-fat palmitic acid-enriched diet with 30%
en from fat. Diet 5 was a high-fat palmitic acid-enriched diet with
40% en from fat. All 5 diets also contained cholesterol at 40mg/1000
kcal of diet. Diets were formulated with different blends of fats
such that in addition to varying the total fat load (at the expense
of carbohydrate), specific fatty acids were also varied. Thus relative
to Diet 1, the extra fat in Diets 2 and 3 was specifically oleic acid,
whereas the extra fat in Diets 4 and 5 (again c.f. Diet 1), was provided
specifically by palmitic acid. Dietary myristic acid (14:0) and linoleic
acid (18:2) was relatively uniform across all 5 diets -0 to 0.50%
en and 5.2 to 5.9% en, respectively. Diets were formulated with the
same nutrient densities (Table 2), and the fatty acid composition
of the diet (Table 3) as fed was verified by GLC5. Irrespective
of the dietary fat load, animals continued to receive a fixed amount
of calories (~52 kcal/animal/ day). Hence the design of the study
allowed for the evaluation of the effects of increasing the dietary
fat load, when only one fatty acid increases. Animals were fed their
respective diets for up to 8 weeks during which time plasma and lipoprotein
lipids as well as in vivo lipoprotein metabolism were evaluated
as detailed in subsequent sections. All procedures and protocols were
in accordance with the University’s Animal Use and Radiation
Safety Committees.
Plasma
and lipoprotein lipid determinations. Individual hamsters were fasted overnight (~16 hours) in hanging cages.
Blood was collected by cardiac puncture (following light anaesthesia
with 50% O2/50% CO2) using an EDTA-wetted syringe,
and transferred to EDTA-containing tubes which were kept on ice. Plasma
was isolated by centrifugation at 1000x g, 10 min, 4°C. Lipoprotein
fractions were isolated either by discontinuous density gradient or
sequential ultracentrifugation as indicated in the text. Cholesterol
and triglyceride concentrations in both plasma and lipoprotein fractions
were determined enzymatically (kit #352 and #336 respectively, Sigma
DiagnosticsRTM, St. Louis, MO).
Lipoprotein
isolations. Prior to isolation of any
lipoprotein, sodium azide, gentamycin sulfate, benzamidine and EDTA
were added to all plasma samples16,17. Plasma, pooled according
to diet, was utilised for separation of lipoproteins using a 5-step
salt gradient by density gradient ultracentrifugation exactly as detailed
by Goulinet and Chapman18. Following ultracentrifugation
at 15°C, 35k for 48 hours in a SW 41.1 rotor, 30 fractions were collected
(a top fraction of 500 mL, and 29 subsequent fractions of
400 mL each). The cholesterol content of each fraction was determined enzymatically,
as well as the density of each fraction as described elsewhere11.
Based on the above cholesterol and density distributions, subsequent
lipoprotein isolations utilised a Ti 50.4 fixed angle rotor to isolate
VLDL+IDL (d<1.02 g/mL), LDL (1.02<d<1.05 g/mL) and HDL (1.05<d<l.18
g/mL) by sequential ultracentri-fugation19. In the rest
of this report the VLDL+IDL fraction will be referred to simply as
VLDL.
Table 2. Caloric content of hamster diets (kcal/
l00g diet)
| Ingredient |
Diet 1
|
Diet 2, 4
|
Diet 3, 5
|
| Casein |
88.8
(24%en)
|
94.8
(24.3%en)
|
101.4
(24.6%en)
|
| Cornstarch |
139
(38%en)
|
103.6
(27%en)
|
64.6
(16%en)
|
| Glucose |
53.2
(14%en)
|
56.8
(15%en)
|
60.8
(15%en)
|
| Cellulose |
15
(4%en)
|
16
(4%en)
|
17.1
(4%en)
|
| Fat |
74.25
(20%en)
|
119.25
(31%en)
|
168.75
(41%en)
|
| Total |
370
|
390
|
413
|
| Cholesterola |
40.5
|
40.5
|
40.5
|
| Mineralsb |
13.5
|
13.7
|
13.8
|
| Vitaminsb |
3.24
|
3.28
|
3.32
|
a mg/1000 Kcal; bg/1000 Kcal
Table 3. Fatty acid composition of hamster
purified diets (%energy).
| Fatty acid |
Diet 1
|
Diet 2
|
Diet 3
|
Diet 4
|
Diet 5
|
| 14:0 |
0.4
|
0
|
0.1
|
0.3
|
0.5
|
| 16:0 |
6.6
|
6.7
|
7.7
|
12.9
|
21.9
|
| 18:1 |
6.9
|
16.5
|
24.8
|
9.5
|
9.9
|
| 18:2 |
5.2
|
5.9
|
5.4
|
5.6
|
5.9
|
Diets were analysed by GLC.
Preparation
of lipoprotein tracers. Six days prior
to commencement of the turnover study, several hamsters from each
group were fasted individually for 16h in hanging cages. Following
anaesthesia with 50%O2/50%CO2, they were exsanguinated
by cardiac puncture. Plasma was harvested following centrifugation,
and LDL (1.02 < d < 1.05 g/mL) and HDL (1.05 < d < 1.
18 g/mL) were isolated from pooled plasma by sequential ultracentrifugation
and washed and concentrated by recentrifugation at their appropriate
densities. Following dialysis (0.15 M NaCl/ lmM EDTA pH 7.4), lipoprotein
protein concentration was determined using Markwell’s modification20
of the Lowry procedure21. The LDL was divided into two
aliquots, which were radiolabeled with Na125 I and Na131I
(Amersham, Chicago, IL) respectively. The 131I-LDL was
subsequently reductively methylated as detailed previously7.
Additionally, an aliquot of HDL was radiolabeled with Na131I.
All three tracers were extensively dialyzed against saline prior to
injection. For all three tracers the intramolecular distribution of
radioactivity was determined7. For both the native 125I-LDL
and methylated 131I-LDL, apo B associated radioactivity
was ~88%, while for 131I-HDL, protein-bound radioactivity
was in excess of 97%.
Protocol
for metabolic studies. Potassium iodide
(0.1 g /l00mL) was added to the drinking water of all hamsters
48 hours before injection of tracers. Hamsters were injected simultaneously
with either 125I-LDL and methylated l3lI-LDL
or l251-LDL and 131I-HDL via the jugular vein.
A 100 mL blood sample was obtained by cardiac puncture at 2 min post-injection.
Subsequent blood samples (100mL) were obtained periodically by
cardiac puncture over the next 48 hours. At each instance the hamsters
were lightly anaesthetised with 50%O2/50%CO2.
All animals continued to have access to KI-supplemented water until
2 days after collection of the last blood sample. All blood samples
were kept on ice until the separation of plasma. Duplicate aliquots
of plasma (20-50 mL) were counted for determination of 125I-
and 131I-associated radio activities.
Kinetic
analyses Plasma 125I- and
131I radioactivity data were bi-exponential for all three
tracers, and were therefore analysed in accordance with a 2-pool model22
and their half-lives and fractional catabolic rates (FCR) were calculated23.
The assumptions and rationale for using the 2-pool model have been
detailed previously7,8. Additionally, the limitations of
the analyses in using HDL concentrations and the FCR for whole HDL,
as a measure of HDL apo A1 metabolism have been discussed8.
Statistical
Analyses All statistical analyses were
performed using a Power Macintosh 6100R TM computer (Apple
Systems Inc., Cupertino, CA) with the Statview 512+TM (Brain
Power Inc., Calabasca, CA) statistical package. Significant differences
were calculated using a one way analysis of variance test. Results
are presented as the mean±SD.
Results
Body weights, plasma cholesterol and triglyceride
concentrations averaged 157 ± 17 g, 149 ± 16 mg/dL and 136 ± 40 mg/dL,
respectively (mean ± SD, n=33) in the hamsters at the end of the 2-week
run-in period.Body weights and plasma lipids
after 6 weeks of feeding the test diets are shown in Table 4. There
were no significant dietary effects on any of the measured parameters.
The 6 week data was not significantly different from the 4 week data
(results not shown), indicating that the animals had achieved a steady-state
with respect to their plasma lipid concentrations. In accordance with
the plasma lipid data, there were no discernible differences in the
lipoprotein cholesterol concentrations measured after sequential ultracentrifugation
of plasma from individual animals (Table 5). For all diets, VLDL-C
averaged ~13% of total lipoprotein cholesterol, while the figures
for LDL and HDL were ~21% and ~66%, respectively.
The 6 week lipoprotein isolations were carried out
by sequential ultracentrifugation and VLDL, LDL and HDL were isolated
at the density intervals d<1.02g/mL, 1.02<d<1.05 g/mL and
1.05<d<l.18 g/mL, respectively. These density cuts were based
on the 4 week lipoprotein isolations carried out by discontinuous
density gradient ultracentrifugation (Figure 1) which also revealed
no diet effects.
Clearance of native LDL, methylated LDL and HDL was
assessed in a subset of 33 animals after ~8 weeks on diet, and the
resulting data is shown in Table 6. Consistent with the plasma lipid
data, there were no dietary influences on the FCR of native LDL, methylated
LDL or HDL. Receptor-independent clearance represented ~48% of total
clearance and thus receptor-dependent clearance accounted for ~52%
of the total clearance. The half-life of the methylated LDL (irrespective
of diet) was significantly longer than the half-life of the native
LDL (21.7 ± 3.6 hrs vs.13.4 ± l.7 hrs, p = 0.0001), indicative of
its longer residence time in the plasma. The half-life of whole HDL
(25.4 ± 3.5 hrs) was significantly longer than the half-life for both
the methylated and native LDL.
Table 4. Body weights and plasma lipid concentrations
after 6 weeks.
| Diet |
BW (g)
|
TC (mg/dL)
|
TG (mg/dL)
|
| #1 (18) |
166±20
|
141±21
|
125±28
|
| #2 (15) |
174±13
|
145±16
|
138±26
|
| #3 (20) |
180±15
|
142±21
|
126±25
|
| #4 (13) |
169±20
|
151±27
|
147±40
|
| #5 (14) |
176±20
|
130±17
|
120±28
|
Values are the mean ± SD of the number of animals
given in parentheses. There was no significant difference between
dietary groups for any of the three parameters (assessed by ANOVA).
Table 5. Plasma and lipoprotein cholesterol
concentrations (mg/dL).
| Diet |
TC
|
VLDL
|
LDL
|
HDL
|
| #1 |
137±13
|
17±4
(13%±2%)
|
33±4
(24%±3%)
|
87±11
(63%±2%)
|
| #2 |
131±16
|
17±4
(13%±2%)
|
28±5
(21%±2%)
|
86±7
(66%±3%)
|
| #3 |
137±8
|
20±6
(14%±5%)
|
27±3
(20%±2%)
|
90±7
(66%±5%)
|
| #4 |
139±30
|
19±8
(13%±3%)
|
29±7
(21%±4%)
|
91±18
(66%±4%)
|
| #5 |
133±30
|
18±13
(13%±6%)
|
31±11
(23%±3%)
|
84±7
(64%±8%)
|
Lipoproteins were isolated by sequential ultracentrifugation
from the plasma of individual hamsters at the following density intervals
- VLDL d<1.02 g/mL; LDL 1.02<d<1.05 g/mL and HDL 1.05<d<1.18
g/m L.
Values are the mean ± SD for 5-6 hamsters per dietary group. Percentage
of total lipoprotein cholesterol in parenthesis.
Discussion
The current study was conducted in order
to evaluate the effects of increasing dietary fat quantity - specifically
by increasing only one fatty acid - on plasma lipids. Theoretically,
the study might have started with a control diet of 40% en (with equivalent
contributions from SFA, MUFA and PUFA) in which fat quantity decreased
to 30% en and 20% en by decreasing either 16:0 or 18:1. However, this
design was not possible given the limitation that we wished to utilise
naturally occurring oils, as opposed to artificially generated triglycerides,
on the assumption that triglyceride structure may affect lipoprotein
metabolism. Thus a 20% control diet was selected in which SFA, MUFA
and PUFA were roughly in a 1/1/1 ratio. With this control diet as
a starting point, it was possible to formulate diets with 30% en or
40% en from fat, in which the excess fat was contributed by either
18:1 or 16:0, but, 14:0 and 18:2 were held constant. Within this design
frame, the results clearly show that within a 20% en to 40% en dietary
fat load, 16:0 and 18:1 can be readily interchanged without having
any effect on plasma total or lipoprotein cholesterol concentrations.
This was further reflected in the in vivo kinetic data, which
revealed no dietary effects on LDL and HDL clearance rates.
Figure 1. Cholesterol and density distribution
of plasma lipoproteins isolated by discontinuous density gradient
ultracentrifugation (DDGUC).
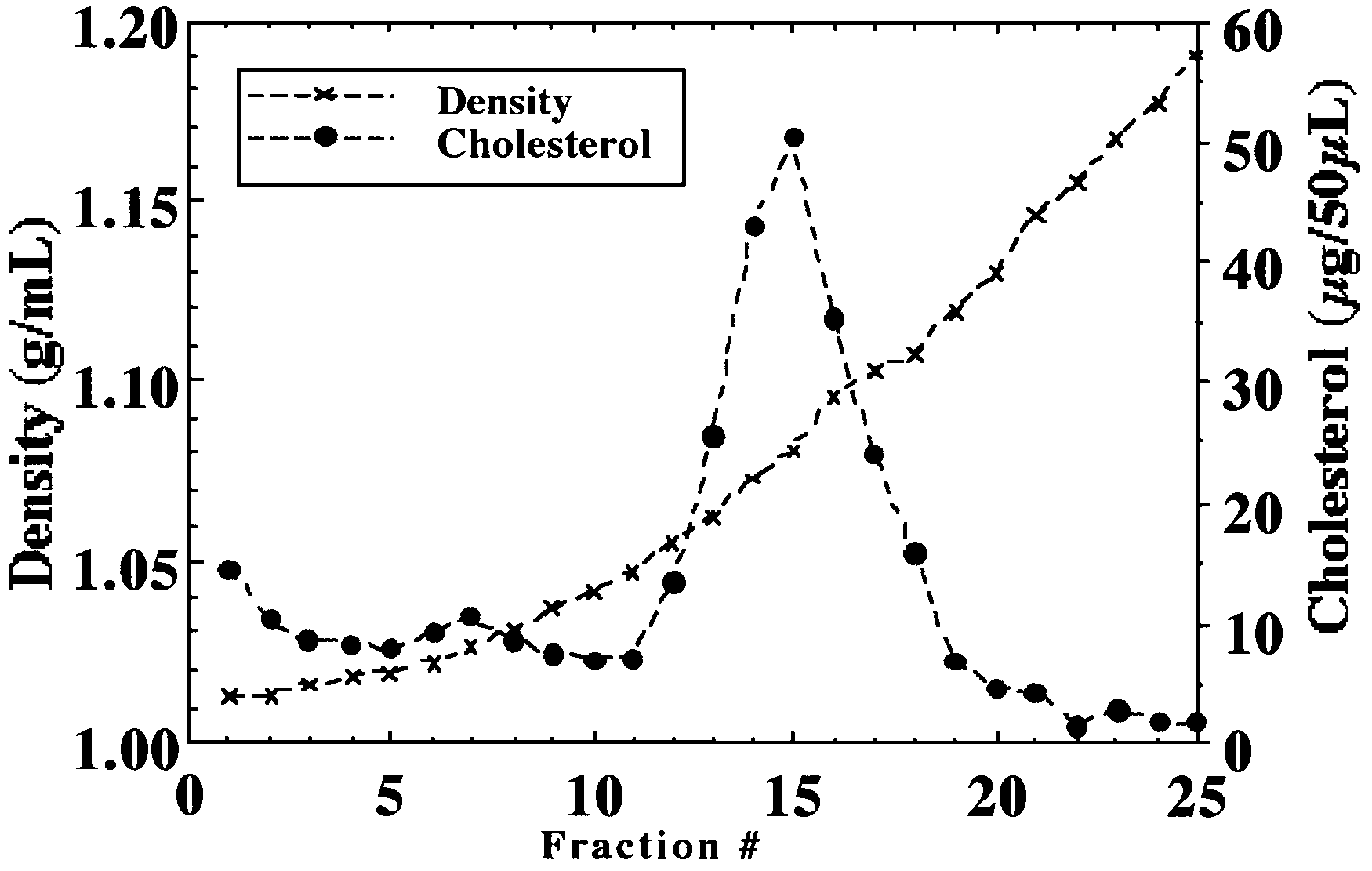
Lipoproteins were isolated by DDGUC and 30 fractions
collected. The cholesterol content of each fraction as well as its
density was determined. Profile shown is from the control diet (Diet
1). Similar results were obtained with the plasma from the other diets.
Table 6. Clearance of native LDL, methylated
LDL and HDL.
| Diet |
|
nat LDL
|
|
|
meth LDL
|
|
|
HDL
|
|
| |
|
FCR
pools/day
|
t 1/2
hrs
|
|
FCR
pools/day
|
t 1/2
hrs
|
|
FCR
pools/day
|
t 1/2
hrs
|
| #1 |
(7)a
|
1.805+0.297
|
14.30±1.81
|
(3)
|
0.89±0.12
|
22.8±2.0
|
(4)
|
0.74±0.09
|
28.3±0.7
|
| # 2 |
(5)
|
1.960±0.518
|
13.81±1.76
|
(2)
|
0.76,0.84
|
23.5,23.1
|
(3)
|
0.89±0.07
|
27.7±6.5
|
| #3 |
(7)
|
2.080±0.672
|
13.50±1.30
|
(3)
|
0.86±0.33
|
23.7±6.6
|
(4)
|
0.95±0.10
|
23.4±2.0
|
| #4 |
(6)
|
1.833±0.317
|
13.63±1.64
|
(3)
|
1.02±0.35
|
20.0±1.9
|
(3)
|
0.79+0.12
|
25.0±2.7
|
| #5 |
(7)
|
2.542±0.898
|
12.04±1.35
|
(3)
|
1.24±0.21
|
19.6±2.4
|
(4)
|
1.08±0.30
|
23.1±1.9
|
| All |
(32)
|
2.056±0.625
|
13.43±1.67
|
(14)
|
0.99±0.27
|
21.7±3.6
|
(18)
|
0.89±0.19
|
25.4±3.5
|
a Number of animals. A total of 33 animals
were injected with 125I-LDL, and either methylated 131I-LDL
(n=15) or 131I-HDL (n=18). One animal from Diet 2 (injected
with native and methylated LDL) died during the course of the turnover.
Thus complete data were available from 32 animals. Values are presented
as mean±SD, except for the diet 2 animals injected with methylated
LDL, for whom individual values are listed. FCR’s and half-lives
were calculated as indicated in the text. One way ANOVA indicated
no significant difference between diets for any of the measured parameters.
For all animals combined, the half life for the native LDL was significantly
lower than the half-life of the methylated LDL and HDL (p=0.0001).
Additionally, the half-life of the methylated LDL was significantly
lower than the HDL half life (p=0.0001).
In previous studies in hamsters2 and guinea
pigs1,24, the effects of decreasing dietary fat quantity
on plasma lipids were dependent largely on the basal fat employed.
In the hamster, plasma LDL decreased when the basal fat was principally
hydrogenated coconut oil (HCO) and fat quantity was decreased from
40% en to 20% en2. While the above effect was attributed
to the SFA content of the diet, the current data suggests that altered
intake of 14:0 and 18:2 may have been responsible, especially as HCO
a) has a very high 14:0 content and b) no 18:2. Although safflower
oil was blended into the HCO-based diet, the levels of 18:2 were considerably
less than the ~5% en employed in the current study. Similarly, in
the guinea pig studies, varying fat quantity between 19% en and 35%
en modulated plasma lipids differentially depending on whether the
basal fat was lard, olive oil or corn oil. However, in each of these
fats the levels of 14:0 or 18:2 were not constant.
The current study which evaluated 16:0 and 18:1 exchanges
revealed no effects on plasma lipids even though diets provided cholesterol
(albeit at low levels). However, the "lack" of cholesterol
was not the factor in determining the response. The hamster when fed
a cholesterol-free purified diet based solely on coconut oil (high
14:0, low 18:2) exhibits a plasma cholesterol almost 60-70 mg/dL higher
than the levels observed when safflower oil (no 14:0, high 18:2) is
the dietary fat source (Pronczuk et al, unpublished). This
differential response is magnified in the gerbil (almost 120 mg/dL),
but a similar study employing the same diets (devoid of cholesterol)
also revealed that increasing fat quantity from 20% en to 30% en to
40% en had no effect on plasma lipids when the excess fat was 16:0
or 18:1. However, plasma lipids increased when the excess fat
was from 14:0 and decreased when the excess fat was from 18:2
(Khosla et al, unpublished).
Thus the results of the current study lend support
to the previous observations from this laboratory7,10,25
that in situations of normal LDL receptor activity, 16:0 and 18:1
can be readily interchanged without compromising plasma lipids provided
14:0 levels are tightly controlled and 18:2 levels are adequate.
While the current design may be difficult to test
using Western type diets in humans, the results may be of relevance
to populations accustomed to low levels of fat intake. In India and
China, dietary fat constitutes ~15% en. With increasing economic prosperity,
fat intake is on the increase in certain sections of the population.
The current data, cautiously extrapolated to humans, would
suggest that if fat consumption increases, it would be prudent to
balance the intakes of 14:0 and 18:2 as fatty acids increase.
Acknowledgment. This study was funded partially by Palm Oil Research Institute
of Malaysia (PORIM).
References
- Fernandez ML, Lin ECK McNamara DJ. Regulation of
guinea pig lipoprotein kinetics by dietary fat saturation. J Lipid
Res. 1992; 33: 97-109.
- Woollett LA, Spady DK, Dietschy JM. Saturated and
unsaturated fatty acids independently regulate low density lipoprotein
receptor activity and production rate. J. Lipid Res. 1992; 33: 77-88.
- Lindsey S, Benattar J, Pronczuk A, Hayes
KC. Dietary palmitic acid (16:0) enhances HDL cholesterol and LDL
receptor mRNA abundance in hamsters. Proc. Soc. Exp. Biol. Med.
1992; 195: 261-269.
- Hayes KC, Pronczuk A, Lindsey S, Diersen-Schade
D. Dietary saturated fatty acids (12:0, 14:0, 16:0) differ in their
impact on plasma cholesterol and lipoproteins in nonhuman primates.
Am J Clin Nutr. 1991; 53: 491-498.
- Pronczuk A, Patton GM, Stephan ZF, Hayes
KC. Species variation in the atherogenic profile of monkeys: Relationship
between dietary fats, lipoproteins and platelet aggregation. Lipids
1991; 26:213-222.
- Khosla P, Hayes KC. Dietary fat saturation
in rhesus monkeys affects LDL concentrations by modulating the independent
production of LDL apolipoprotein B Biochim Biophys Acta. 1991; l083:
46-56.
- Khosla P, Hayes KC. Comparison between
dietary saturated (16:0), monounsaturated (18:1) and polyunsaturated
(18:2) fatty acids on plasma lipoprotein metabolism in cebus and
rhesus monkeys fed cholesterol-free diets. Am J Clin Nutr. 1992;
55: 51-62.
- Khosla P, Hayes KC. Dietary palmitic acid raises
plasma LDL cholesterol relative to oleic acid only at a high intake
of cholesterol. Biochim. Biophys. Acta. 1993; 1210: 13-22.
- Hayes KC, Khosla P. Dietary fatty acid
thresholds and cholesterolemia. FASEB J. 1992; 6: 2600-2607.
- Pronczuk A, Khosla P, Hayes KC. Dietary
myristic, palmitic and linoleic acids modulate cholesterolemia in
gerbils. FASEB J. 1994; 8: 1191-1200.
- Pronczuk A, Khosla P, Hajri T, Hayes KC. Plasma
lipids are affected similarly by dietary lauric and palmitic acid
in gerbils and monkeys. Lipids 1995; 30: 1157-1161.
- Ng TKW, Hayes KC, de Witt GF, Jegathesan
M, Satgunasingham N, Ong ASH, Tan DT. Dietary palmitic and oleic
acids exert similar effects on serum cholesterol and lipoprotein
profiles in normo-cholesterolemic men and women. J Am Coll Nutr,
1992; 11: 383-390.
- Sundram K, Hayes KC, Siru OH. Dietary
palmitate lowers plasma LDL cholesterol in comparison to a laurate-myristate
combination in normocholesterolemic humans. Am J Clin Nutr. 1994;
59: 841-846.
- Sundram K, Hayes KC, Siru OH Both dietary
18:2 and 16:0 may be required to improve the serum LDL/HDL cholesterol
ratio in normo-cholesterolemic men. J Nutr Biochem. 1995; 6: 179-187.
- Choudhury N, Tan L, Truswell, AS. Comparison of
plamolein and olive oil: effects on plasma lipids and vitamin E
in young adults. Am J Clin Nutr 1995; 61: 1043-1051.
- Schumaker VN, Puppione DL. Sequential
flotation ultracentrifugation. Methods Enzymol. 1986; 128: 155-170.
- Edelstein C Scanu AM. Precautionary measures
for collecting blood destined for lipoprotein isolation. Methods
Enzymol 1986;128:151-155.
- Goulinet S Chapman MJ. Plasma lipoproteins
in the golden Syrian hamster (Mesocricetus auratus): heterogeneity
of apoB- and apoA-1-containing lipoproteins. J Lipid Res. 1993;
34: 943-959.
- Havel RJ, Eder HA Bragdon JH. The distribution
and chemical composition of ultracentrifugally isolated lipoproteins
in human serum. J Clin Invest. 1955; 34: 1345-1353.
- Markwell MAK, Haas SM, Bieber LL, Tolbert.
A modification of the Lowry procedure to simplify protein determination
in membrane and lipoprotein samples. Anal Biochem 1978; 87: 206-210.
- Lowry OH, Rosebrough NJ, Farr AL, Randall.
Protein measurement with the Folin phenol reagent. J Biol Chem.
1951; 193: 267-275.
- Matthews CME. The theory of tracer experiments
with 131I-labeled plasma proteins. Phys Med Biol. 1957; 2: 36-53.
- Kushwaha RS, Hazzard WR. Catabolism of
very-low-density lipo-proteins in the rabbit. Effect of changing
composition and pool size. Biochim Biophys Acta. 1977; 528: 176-189.
- Lin ECK, Fernandez ML, Tosca MA, McNamara
DJ. Regulation of hepatic LDL metabolism in the guinea pig by dietary
fat and cholesterol. J Lipid Res 1994; 35: 446-457.
- Hayes KC, Pronczuk A, Khosla P. A rationale
for plasma cholesterol modulation by dietary fatty acids: modelling
the human response in animals. J Nutr Biochem, 1995; 6: 188-194.
Dietary oleic and palmitic acid
exert similar effects on plasma lipids and lipoprotein metabolism
in hamsters fed purified diets with
low cholesterol but different quantities of fat
Pramod Khosla, Andrzej Pronczuk, Tahar Hajri and KC Hayes
Asia Pacific Journal of Clinical Nutrition (1997) Volume 6, Number
1: 26-30


Copyright © 1993 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
 to the top
to the top