1000
Asia Pacific J Clin Nutr (1996) 5: 96-99
Asia Pacific J Clin Nutr (1996) 5: 96-99
Effect
of Cocos
nucifera and red chilli on intestinal b-glucuronidase
and mucinase activity in experimental colon cancer
N Nalini1 MSc, S Chitra1
MSc, K Sabitha1 MSc, P Viswanathan2
MD and Venugopal P Menon1 MSc PhD
- Department of Biochemistry, Annamalai
University, Annamalainagar 608 002, Tamil Nadu, India
- Department of Pathology, Rajah
Muthiah medical College,Annamalai University, India
Effect of Cocos nucifera and red chilli on intestinal
B-glucuronidase and faecal mucinase activity, was studied in rats
given 1 ,2-dimethylhydrazine (DMH) . The average weight gain by
the animals given coconut kernel was more than the DMH and chilli
treated groups. The activity of B-glucuronidase decreased in the
kernel groups, in most of the tissues studied, as compared to the
DMH and chilli treated groups. A similar pattern was observed in
the case of mucinase. Morphological studies showed that the number
of visible malignant tumours decreased in the colon and intestine
of the animals, when their diet was supplemented with coconut kernel.
Histopathological studies also showed that the animals had fewer
papillae, lesser infiltration into the sub-mucosa and lesser changes
in the cytoplasm with decreased mitotic figures, when kernel was
included in the diet. Coconut kernel, thus reduced the mutagenic
and carcinogenic effect of chilli and DMH respectively.
Introduction
Dietary intervention can protect humans from a variety
of diseases and has led to the formulation of a number of explanatory
hypotheses, several of which involve the bacteria of the lower intestine1-5.
These bacteria are capable of a wide variety of metabolic activities
including production of toxic metabolites, transformation of bile
acids, reduction and hydrolysis of drugs that may positively act as
carcinogens and/or co-carcinogens.
b-glucuronidase
and mucinase are two important enzymes which reflect the activity
of these bacteria. Mucinase is the enzyme which hydrolyses the protective
mucins and b-glucuronidase hydrolyses biliary glucuronides. If glucuronide hydrolysis
is a rate-limiting step in this process, then the levels of microbial
b-glucuronidase in the colon may influence the risk of colon cancer.
Among the spices, red-chilli is consumed in large
1000 quantities in different parts of India6,7. Coconut
kernel is also an important constituent of Indian food. In vitro
studies have shown that red chilli and its irritative phenolic compound,
capsaicin, known to have an established structure of N-(4-hydroxy-3-methoxy
benzyl)-8-methyl trans-6-enamide6,7 to be a mutagenic,
carcinogenic and tumour promoting agent10,11. Since Cocos
nucifera forms an important constituent of Indian food, we have
studied the effect of coconut kernel on 1, 2-dimethylhydrazine (DMH)
induced colon carcinoma and also its effect, in the presence of red
chilli.
Materials and methods
Wistar male albino rats bred in the Animal House of
Rajah Muthiah Medical College, Annamalai University, weighing 120-150
g were divided into 7 groups of 10 rats each. They were all fed a
commercial diet (Lipton Lever Limited) containing 20% peanut oil .
Water was given ad libitum.
Group 1 were control rats, group 2 were rats fed fresh
coconut kernel (30%), group 3 were rats administered DMH*, group 4
were rats fed red chilli powder (8mg/day/ 100g body weight in water),
group 5 chilli + DMH, group 6 fresh coconut kernel + red chilli, group
7 fresh coconut kernel + chilli + DMH.
The fat intake by the animals in groups 2, 6 and 7
were adjusted, so that it was similar to the fat intake in groups
3, 4 and 5. The caloric intake of animals in groups 3, 4 and 5 were
also similar to that of 2, 6 and 7. p-nitrophenyl b-D-glucuronide, mucin and 1,2-dimethylhydrazine
were purchased from Sigma Chemical Co, St. Louis, MO, USA. All the
other chemicals used were of analytical grade and were purchased from
SD Fine Chemicals, Bombay, India.
DMH was administered as reported earlier12.
After 15 weeks, the DMH injection was discontinued and the rats were
given only commercial diet. The animals were observed daily and weighed
every week. At the end of 30 weeks, fresh faecal pellets were collected
and the activity of mucinase was estimated by the method of Shiau
and Chang13. The rats were then sacrificed and the neoplasms
in the intestine and colon were counted after cutting open the tissues
longitudinally taking care not to disturb the tumours. Part of the
tissues were sent for histopathological examination. The rest of the
tissues and colon contents (bacterial contents) were transferred to
ice cold containers, for measuring the activity of b-glucuronidase14. Protein was
estimated by the method of Lowry et al15.
Results obtained are expressed as mean ± SE from 6 rats in each group. The
statistical significance of difference in means was analysed by Student’s
t-test. A one way analysis of variance (ANOVA) was also determined16.
Results
Table 1 shows the incidence of colon and intestinal tumours in
all the 7 groups. The values expressed are the sum of about 30 surviving
rats from different experiments. The incidence and number of tumours
decreased when coconut kernel was supplemented in the diet.
The macroscopy and light microscopic observations
(histopathological) of the colon of rats in different groups are given
in Table 2. The table shows that when kernel was supplemented in the
diet the animals showed fewer papillae, lesser infiltration into the
submucosa and less changes in the cytoplasm with dec 1000 reased mitotic
figures.
Figure 1 gives the average growth rate of the animals
in the various groups. It was observed that the weight gained by the
control group > kernel group > kernel + chilli + DMH > kernel
+ chilli > chilli + DMH > chilli > DMH, even though the average
food intake by the animals of the various groups were more or less
similar. The energy intake was the same in all the groups. Figure
2 shows the intestine and colon of rat.
b-glucuronidase
activity showed a significant increase in the DMH, chilli and chilli
+ DMH groups when compared with the control rats (Table 2).
Table 1. Incidence
of colon and intestinal tumours.
| Rat group |
Rats with tumours/ total rats
|
Incidence of colon tumours (% )
|
Tumours in colon/ tumour- bearing rat
|
Tumours in intestine/ tumour-bearing rat
|
| Group 1 Control |
Nil
|
Nil
|
Nil
|
Nil
|
| Group 2 Kernel
|
Nil
|
Nil
|
Nil
|
Nil
|
| Group 3 DMH |
27/30
|
90.0
|
26
|
12
|
| Group 4 Chilli
|
25/30
|
83.3
|
17
|
8
|
| Group 5 Chilli
+ DMH |
28/30
|
93.3
|
34
|
15
|
| Group 6 Kernel
+ chilli |
6/30
|
20.0
|
2
|
Nil
|
| Group 7 Kernel
+ chilli + DMH |
22/30
|
73.3
|
12 3
|
|
|
Table 2. Histopathological changes in the colon.
| |
Group 2
|
Group 3
|
Group 4
|
Group 5
|
Group 6
|
Group 7
|
| Macroscopy |
|
|
|
|
|
|
| 1. Size
|
-
|
< 1000 td valign="top" width="14%">
2 cm
1 cm
|
2 cm
|
< 0.5 cm
|
1 cm
|
| 2. Margin
|
-
|
Well defined
|
Defined
|
Ill defined
|
Ill defined
|
Ill defined
|
| 3. Nature
|
-
|
Pedunculated
|
Pedunculated
|
Pedunculated
|
Sessile
|
Sessile
|
| Microscopy |
|
|
|
|
|
|
| 1. Transitional
zone with foci of dysplasia |
-
|
Not present
|
Focal areas of dysplasia
|
Present
|
Occasional dysplastic gland
|
Present
|
| 2. Inflammatory
cell infiltrate into the mucosa |
-
|
Mixed population
|
Mixed population
|
Mixed population
1000 |
Mixed population
|
Mixed population
|
| 3. Lymphoid
aggregates in the submucosa |
-
|
Not observed
|
Not observed
|
Occasional lymphoid aggregate
|
Not observed
|
Not observed
|
| 4. Papillary
pattern |
-
|
Large number of papillae
|
No papillae
|
Few papillae
|
No papillae
|
Significant number of papillae
|
| 5. Mucin
secretion |
-
|
Few glands dilated, filled with mucin
|
-
|
Glands dilated and filled with mucin
|
-
|
Some glands filled with secretion
|
| 6. Infiltration
in the submucosa |
-
|
Observed
|
-
|
Several areas of infiltration
|
-
|
Occasional focus of infiltration seen
|
| 7. Cell
morphology |
|
|
|
|
| |
| i) Nuclear
pleomorphism |
-
|
Marked
|
Less severe
|
Marked
|
Less severe
|
Marked
|
| ii) Nucleoli
|
-
|
Prominent
|
Less prominent
|
Prominent
|
Less prominent
|
Prominent
|
| iii) Cytoplasm |
-
|
Scanty
|
Moderate
|
Scanty
|
Moderate
|
Scanty
|
| iv) Mitotic
figures |
-
|
Numerous
|
Present
|
Numerous
|
Not present
|
Present
|
| Others |
|
|
|
|
|
|
| 1. Vascular granulation
|
Increased vascular granulation
|
Not seen
1000 |
Not seen
|
Vascular granulation (not very prominent)
|
Not seen
|
Vascular granulation present
|
| Figure 1.
Average growth rate. |
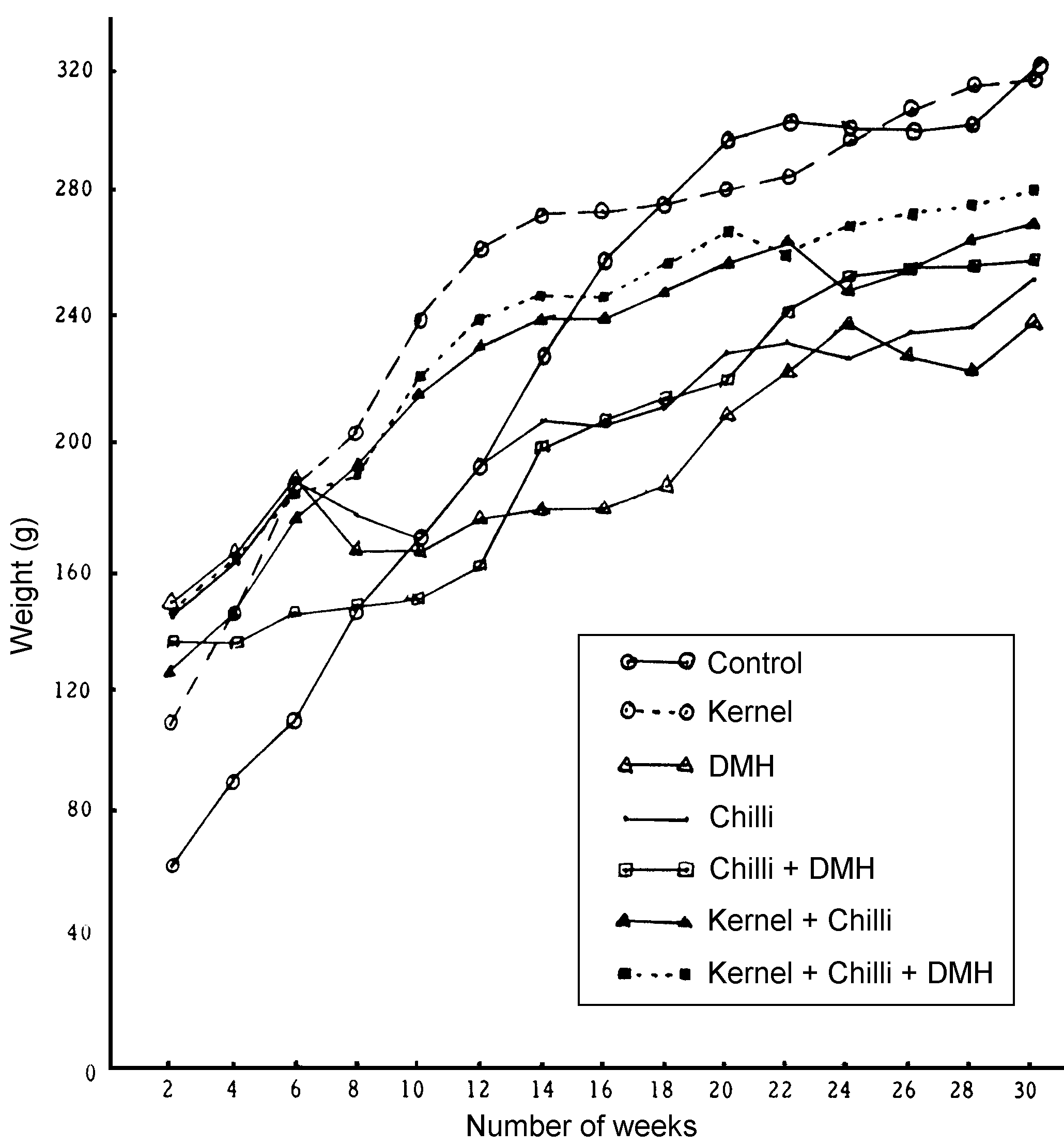 |
| Figure 2.
Rat, digestive system removed from body. |
 |
Table 3. b-glucuronidase activity (mg of p-nitrophenol
liberated/ hr/ g protein).
| |
Group1
|
Group2
|
Group3
|
Group4
|
Group5
|
Group6
|
Group7
|
F-ratio
|
| Distal colon |
55.84±6.916
|
40.317±5.862a
|
108.74±9.275a
|
44.76±4.350b
|
83.40±14.500
|
42.15±3.101a
|
61.60±2.184 NS
|
68.26*
|
| Proximal colon |
47.25±3.783
|
30.00±2.484a
|
47.36±3.730 NS
|
50.97±5.449 NS
1000 |
56.91±7.269b
|
47.95±2.020 NS
|
59.50± 3.301a
|
29.78*
|
| Distal intestine |
53.66±6.245
|
42.01±3.357b
|
54.26±9.501 NS
|
85.767±12.810a
|
80.88±8.648a
|
47.75±3.416 NS
|
61.525± 2.729b
|
28.55*
|
| Proximal intestine |
53.96±7.636
|
39.00±2.755a
|
51.01±7.00 NS
|
79.53±12.591a
|
65.34±3.959a
|
48.85±3.261 NS
|
56.183± 2.874 NS
|
20.69*
|
| Liver |
99.95±8.286
|
85.60±2.137a
|
145.12±12.981a
|
96.08±12.595 NS
|
131.63±23.790b
|
91.00±2.156 NS
|
119.43± 4.525a
|
19.22*
|
| Colon contents |
102.13±8.238
|
71.70±4.093a
|
112.18±13.732 NS
|
149.00±24.390a
|
173.98±15.330a
|
121.40±8.400b
|
160.20±9.640a
|
6.29*
|
Values are mean ± SE from 6 rats in each group. Group
1 has been compared with groups 2-7.
a: p < 0.01 b: p <0.05 NS - Not significant *ANOVA - Significant
at 1% level.
Table 4. Mucinase activity (n moles of glucose
liberated/min/mg protein)
| |
Group1
|
Group2
|
Group3
|
Group4
|
Group5
|
Group6
|
Group7
|
F-ratio
|
| Colon contents |
1.997±0.106
|
1.097±0.191a
|
3.065±0.420a
|
2.693±0.328a
|
4.518±0.663a
|
1.990±0.293 NS
|
3.380±0.509a
|
44.61*
|
| Faecal contents |
1.921±0.261
|
3.480±0.569
|
5.580±0.625a
|
4.300±0.652a
|
7.3 1000 60±0.916a
|
3.500±0.443a
|
4.870±0.834a
|
43.72*
|
Values are mean ± SE from 6 rats in each group. Group
1 has been compared with groups 2-7.
a: p0.01; b: p<0.05; NS - Not significant; *ANOVA - Significant
at 1% level
In the chilli treated animals the b-glucuronidase levels increased
significantly in the colon, intestines and liver, and also in the
colon contents (bacterial) when compared with kernel + chilli. Similarly,
when the chilli + DMH group was compared with the kernel + chilli
+ DMH group, the b-glucuronidase level fell significantly
in the latter group in the distal colon, intestines, liver and also
in the colon contents (bacterial).
A similar pattern was noted in the case of mucinase
(Table 3). The chilli group showed a significant increase when compared
with the kernel group, and kernel + chilli group.
The kernel + chilli + DMH group showed a significant
fall in the activity of mucinase both in the colon contents as well
as in the faecal contents when compared with chilli + DMH group.
The F-value showed that there was a significant difference
between and the within the groups at 1% level in all the parameters
studied.
Discussion
Treatment with red chilli, DMH and coconut kernel
brings about profound alterations in the activity of both b-glucuronidase and mucinase. Chilli
treated rats and those given DMH showed increased incidence of tumours
both in the colon and intestine. When coconut kernel was included
in the diet, the incidence of tumours decreased, the size of the tumours
(visible) were significantly reduced and were more or less diffused.
Histopathological studies showed a great degree of
variation in the different groups. In the case of kernel treated control
animals there was vascularisation in the colon, but the colon was
otherwise normal. The chilli group showed the size of the tumour to
be about 1 cm, pedunculated with defined margin. It showed areas of
dysplasia which were less severe, the nucleoli was less prominent,
moderate cytoplasm and with mitotic figures. In the DMH treated group,
the size of the tumour was around 2 cm, pedunculated, with well-defined
margin, with large number of papillae and an invasive adenocarcinoma,
which showed marked pleomorphism. The nucleoli was also very prominent,
with scanty cytoplasm and numerous mitotic figures. In the chilli
+ DMH group, the size of the tumour was more than 2 cm. There was
a transitional zone with areas of marked dysplasia and infiltrating
adenocarcinoma. The nucleoli was also prominent. In the kernel + chilli
group, the size of the tumour was less than 0.5 cm, with ill defined
margin, sessile with occasional dysplasia. Nuclear pleomorphism was
less severe, nucleoli was less prominent with moderate cytoplasm.
In kernel + chilli + DMH group, the size of the tumours was 1 cm,
sessile with ill defined margin, had a significant number of papillae
with few glands filled with mucin and showed occasional infiltration
into the submucosa. Nuclear pleomorphism was marked with prominent
nucleoli scanty, cytoplasm and vascular granulation. The vascular
granulation observed in the kernel group may be a protective mechanism,
by which the animal tries to r 1000 esist the invasion of the tumour
into the deeper layer.
Glucuronide formation is a major detoxification mechanism
in mammals17. Many exogenous compounds that are excreted
in the bile as glucuronide conjugates are deconjugated by bacterial
b-glucuronidase and modified further by intestinal bacteria in the large
bowel17,18. The activity of this microflora is affected
by diet, ie, they can alter the biological activity, toxicity , excretion
and reabsorption of many of the exogenous and endogenous compounds
which are considered as carcinogens and/or co-carcinogen metabolites19.
Studies have also shown, b-glucuronidase to be a key enzyme in the activation of DMH metabolites
to carcinogens20. These substances can trigger the formation
of neoplastic changes in the colon and intestine21. The
composition of coconut kernel is approximately: fibre 3.1%, protein
3.6%, fat 38.1%, digestible carbohydrates 9.1% and the rest moisture.
The inclusion of coconut kernel in our study significantly decreased
the activity of this enzyme in the presence of chilli or DMH, or both,
emphasising the protective role of the kernel.
Mucins are glycoproteins consisting of a large number
of carbohydrate side chains attached to a protein core. They serve
as a source of energy for the intestinal bacteria and are consequently
degraded by them21-23. Supplying the microflora with fermentable
dietary fibre (ie, coconut kernel) may permit them to use these substrates
preferentially. Thus, the treatment with coconut kernel
showed a decrease in the activity of mucinase, while chilli and DMH treatment
showed an increase.
Thus our studies show that inclusion of coconut kernel
in the diet, results in alteration in the intestinal and colon tissue
as well as in the colon microflora. Biochemically the activity of
b-glucuronidase and mucinase is decreased, while histopathologically the
degree of invasion by the tumour is controlled in the colon. Macroscopically
also the number of tumours as well as the tumour size is reduced.
Acknowledgment
The authors wish to thank Mr Arumuga Perumal and Mr
Krishnaswamy, for their technical assistance.
Chinese abstract
References
- Dales LG, Friedman GD, Wry HK, Grossman S, William
SR. A case control study of relationships of diets and other traits
to colorectal cancer in American blacks. Am J Epidemiol. 1979; 109:
132-144.
- Eastwood MA. Dietary fiber in human nutrition.
J Sci Food Agric. 1974; 25: 1523-1527.
- Finegold SM, Attebery HR, Sutter VL. Effect of
diet on human fecal flora. Comparison of Japanese and American diets.
Am J Clin Nutr. 1974; 27: 1456-1469.
- Modan B, Barell V, Lubian F, Moan M. Dietary factors
and cancer in Israel. Cancer Res. 1975; 35: 3503-3506.
- Nigro ND, Bull AW, Klopfer BA, Pak MS, Campbell
RL. Effect of dietary fiber on azoxymetane induced intestinal carcinogenesis
in rats. J. Natl. Cancer Inst. 1979; 62: 1097-1102.
- Suzuki T, Iwai K. Constituents of red chilli species.
Chemistry, Biochemistry, Pharmacology and Food Science of the pungent
principle of capsicum species. Alkaloids. 1984; 23: 227.
1000
- Sambaiah, Srinivasan K. Influence of spices and
spice principles on hepatic mixed function oxygenase system in rats.
Ind J Biochem & Biophys. 1989; 26: 254-258.
- Nelson EK, Dawson LE. The constitution of Capsaicin,
the pungent principle of Capsaicin. J Am Chem Soc. 1923; 45: 2170-2181.
- Crombie L, Dandegaonker SH, Simpson KB. Amides
of vegetable origin. Part A: Synthesis of Capsaicin. J Chem Soc.
1955; Jan-Mar: 1025-1027.
- Nagabhushan M, Bhide SV. Mutagenicity of chilli
extract and Cap-saicin using short-term tests. Environ. Mutagen.
1985; 7: 881-888.
- Joth B, Rogan E, Walker B. Tumourgenity and mutagenicity
studies with Capsaicin of hot peppers. Anticancer Res. 1984; 4:
117-120.
- Marianne F, Chen SCD, Li Tsun Chen, Worth Boyce
H Jr. The effect of endotoxins on 1,2-dimethylhydrazine induced
colonic tumour in rats. Cancer, 1990; 65: 1748-1752.
- Shiyeu Shiau, George Chang. Effects of dietary
fiber on fecal mucin-ase and b-glucuronidase activity
in rats. J Nutr 1983; 113: 138-144.
- Kawai Y, Anno K. Mucopolysaccharide degrading enzymes
from the liver of the squid. Omastrephes Solanipacifus. Biochem
Biophys Acta. 1971; 242: 428-436.
- Lowry OH, Rosebrough WJ, Farr AL, Randall RJ. Protein
measure-ment with the Folin’s Phenol reagent. J Biol Chem.
1951; 193: 265-275.
- Ya Lun Chou. In: Statistical Analysis. Experimental
Design and the Analysis of Variance. Holt-Rinehart and Winston Publication,
New York, 1975: 340-355.
- Hill MJ, Draser BS, Aries V. Bacteria and aetiology
of cancer of the large bowel. Lancet, 1971; 1: 95-100.
- Reddy BS, Mangat S, Weisburger JH, Wynder EL. Effect
of high risk diets and colon carcinogenesis on intestinal mucosal
b-glucuronidase activity in the F344 rats. Cancer Res. 1977; 37:
3533-3536.
- Reddy BS, Weisburger JH, Wynder EL. Fecal bacterial
glucuron-idase: Control by diet. Science, 1974; 183: 416-417.
- Weisburger JH, Williams GM. Metabolism of chemical
carcinogens. In: FF Becker (ed.) Cancer, A Comprehensive Treatise,
1975; Vol. I, 185-234.
- Hoskins LC, Zambcheck N. Bacterial degradation
of gastrointestinal mucins. Gastroenterology, 1968; 54: 210-217.
- Miller RS, Hoskins LC. Mucin degradation in human
colon eco-systems. Gastroenterology. 1984; 81: 759-765.
- Salvers AA, Vercellotti JR, West SEM, Wilkins TD.
Fermentation of mucin and plant polysaccharides by strains of bacteroides
from the human colon. Appl. Environ. Microbiol. 1977; 33: 319-322.

Copyright © 1996 [Asia Pacific Journal of Clinical Nutrition]. All
rights reserved.
Revised:
January 19, 1999
.
0