1000
Asia Pacific J Clin Nutr (1996) 5: 31-35
Asia Pacific J Clin
Nutr (1996) 5: 31-35
Upper
gastrointestinal tract disease and probiotics
John Lambert, 1 MBBS, PhD FRACP and Ron Hull, 2 PhD
1. Director of Medicine and Gastroenterology
2. Food Microbiologist Department of Medicine Mornington Peninsula
Hospital, Victoria, Australia
Diseases of the oropharynx, oesophagus, stomach
and duodenum are common. This review discusses the microflora
of the upper gastrointestinal tract with particular reference
to lactic acid bacteria and the effect of acid suppression. Probiotics
can survive in these sites and evidence is presented for potential
roles in disease prevention and treatment, particularly with regards
to peptic ulcer disease, Helicobacter pylori infection
and gastric cancer.
Introduction
Diseases of the upper gastrointestinal tract including
the oropharynx, oesophagus, stomach and duodenum are a common cause
of human mortality and morbidity. These diseases may be iatrogenic
or may occur as primary processes. Alterations in the microflora at
these sites may be partly or wholly responsible for development of
disease.
This review discusses the microflora of the upper
gastrointestinal tract, with particular reference to the lactic acid
bacteria group (LAB). The role of probiotics in the prevention and
treatment of diseases will be reviewed.
Microbiology
of the upper gastrointestinal tract
The normal microflora of the mouth, stomach and duodenum
are a rich ecosystem of enormous complexity containing a large number
of species of bacteria1. The oesophagus and mouth have
similar bacterial populations. In the fasting state the stomach and
duodenum contain very few micro-organisms which are mainly derived
from the oral cavity and throat. The total population and species
show dramatic variations along the gastrointestinal tract with the
highest concentrations in the colon. The microflora of normal gastric
juice is shown in Table 1 as observed in 322 samples of gastric juice from normal subjects. As
is evident, the spectrum of organisms grown from the gastric juice
is similar to that which normally inhabits the mouth, pharynx and
oesophagus.
| During fasting the gastric juice
contains only small numbers of bacteria and yeasts usually only
about 102 to 105 /ml. The predominant bacterial
groups found in the stomach and duodenum include Streptococci,
Lactobacillus sp, Veillonella sp and Clostridium perfringens.
After a meal the 1000 bacterial counts in gastric juice increase
100 to 1000-fold2,3. This increase in transient with
return to baseline levels within 1 to 2 hours as a result of
a decrease in gastric juice volume and pH as well as the effects
of gastric motility.
A wide variation of bacterial types occurs among
individuals, however the number of species and population of
bacteria are relatively stable in healthy adults. Within the
upper gastrointestinal tract the normal established "resident"
bacterial microflora may be altered by bacteria introduced into
the body as a normal part of food ("transient" microflora)
or as contaminants ("accidental" microflora). In the
upper gastrointestinal tract these transient bacteria have a
much greater effect on the resident microflora because of the
lower numbers of the latter being present.
In spite of its stability the intestinal microflora
can vary enormously in the stomach and duodenum dependent on
host factors such as level of gastric acid secretion2,3,
bile salts, and mucous in the intestinal wall. In addition medications,
diet, infections, age, stress and climate can also alter the
microflora4. The contents of microflora may also
be influenced by bacterial interaction such as antagonism or
symbiosis. Adaptation of intestinal microflora can occur to
most substances that enter the intestines from the oral tract
or via the biliary system. This adaptation occurs within several
days with the ability of intestinal microflora to metabolise
these substances. Gastric acid inhibits the growth of micro-organisms,
with the stomach of patients having no acid showing an increased
number of bacteria2,5. In these subjects counts of
bacteria of between 106 to 107/ ml. have
been observed. In subjects with no gastric acid (achlorhydria)
the flora is similar to that found in the colon with 50% or
more of patients having coliforms, Bacteroides or other colonic
type2,6. After gastric resective surgery, which is
associated with a decrease in gastric acid, a change in the
bacterial counts are also observed with 10,000 fold higher levels
noted in some subjects. In addition to the higher bacterial
counts an increase in coliforms is also observed6,7.
|
Table 1.
Microflora of normal gastric juice (%
of normal subjects with bacterial organisms*)
| Organism |
% of Patients
|
| Staphylococcus
epidermidis |
61
|
| Streptococcus
mitis |
59
|
| Yeasts (Candida
albicans and others) 1000 |
53
|
| Lactobacillus
spp. |
50
|
| Streptococcus
salivarius |
50
|
| Neisseria
spp. |
37
|
| Micrococcus
spp. |
35
|
| Corynebacterium
spp. |
33
|
| Staphylococcus
auerus |
16
|
*modified from reference 5
|
A number of powerful acid suppressant drugs are now
available with a statistically significant relationship between the
gastric luminal pH and the number of organisms in gastric juice in
patients taking these agents5. As the pH of gastric juice
increases a plateau is reached at about pH 5 to 6 in the median bacterial
counts which peak at between 106 to 108/ ml.
It has been suggested that the concentration of carcinogenic N-nitroso
compounds are increased in gastric juice after antisecretory drugs.
Some authors however have found either no change or a decrease in
vivo nitrosation as intragastric pH rises5.
Lactic acid bacteria (LAB) including Lactobacillus,
Lactococcus, Pediococcus, Leuconostoc and Bifido-bacterium
are found throughout the gastrointestinal tract. The predominant population
of lactic acid bacteria in the upper gastrointestinal tract are Lactobacillus
species. Lactobacilli may colonise the mucosal surface of the duodenum
as well as the stomach. For this to occur they must possess certain
properties including adhesion, competitive exclusion ability and bacterial
inhibitor production.
Probiotics
in the upper gastrointestinal tract
Probiotics, that is live microbial food supplements
beneficially affecting the host by improving its microbial balance,
can survive passage through the stomach and duodenum. Both Bifidobacterium
species and Lacto-bacillus species are capable of transiting
the oesophagus, stomach and duodenum in normal subjects. Gastric acid
does affect LAB. However, survival of bifidobacteria in fermented
milk products occurs in vitro and in vivo for up to
3 hours at a pH of 38. Similarly lactobacilli can survive
similar acidic conditions of around pH 4 for several weeks in vitro.
Diseases of the upper gastrointestinal tract
Diseases of the oesophagus, stomach and duodenum occur
in a high proportion of adults with the lifetime incidence of peptic
ulcer disease of 10%, gastro-oesophageal reflux disease of 25%, indigestion
of unknown cause (non-ulcer dyspepsia) of 20% in subjects living in
the Western world. Gastric cancer varies considerably on a geographic
basis with rates of between 4 to 80 per 100,000 of the population
per year.
The important problem of peptic ulcer disease is now
known to be attributed to a bacterial pathogen, H. pylori,
in conjunction with gastric acid and ulcerogenic drugs. H. pylori
is a gram negative which colonises the gastric mucosa and upper duodenum
and causes long-term histological inflammation in all infected subjects8.
The bacterium is found within gastric mucus and on the surface of
gastric epithelial cells. This organism is transmitted between humans
via oral/oral or faecal/oral spread and once colonisation occurs it
results in an inflammatory process which is sustained for life.
The accompanying inflammation of the stomach may remain
stable or along with other bacterial and environmental factors, gastric
or duodenal ulcer disease, or less commonly gastric malignancy (carcinoma
and certain forms of lymphoma) will develop. H. pylori infects
between 30 to 80% of the world’s adult population with the prevalence
higher in low socio-economic groups, institutionalised individuals,
and amongst family members of infected subjects. The high prevalence
of this disease and its associated pathology reveals that this is
the most common infectious disease worldwide and its control would
result in marked diminution in mortality and morbidity from diseases
of the upper gastrointestinal tract.
Current therapies to eradicate this infection relate
to the use of antimicrobial agents in combination with a bismuth compound
or a potent acid suppressing agent8. These agents are effective
in 80-95% of subjects however side effects, particularly related to
nausea, abdominal pain and diarrhoea, effect up to 15% of subjects.
Gastric cancer is a cause of high mortality in individuals
who develop the disease with the distribution worldwide varying considerably9.
H. pylori is classified by the WHO as a biological carcinogen
causing gastric cancer. A strong correlation between the prevalence
of H. pylori in the community and mortality from gastric cancer
is observed. Moreover, a high prevalence of H. pylori infection
occurs in subjects with the disease. In experimental animals co-infection
with Helicobacter results in increased susceptibility to chemical
carcinogens inducing gastric cancer. Long-term infection with H.
pylori, particularly that which is acquired prior to adolescence,
appears to increase the susceptibility to develop gastric cancer.
Gastric mucosal changes of atrophy and intestinal metaplasia predispose
to the development of malignancy and are a function of the duration
of infection. Other factors including dietary agents, chemical carcinogens
(nitrites and N-nitroso compounds) and hypoacidity all appear to be
co-factors in development of cancer.
Probiotics
in prevention and treatment of upper gastrointestinal tract disease
| Probiotic bacteria have important
properties that would make them potentially useful in the treatment
and prevention of upper gastrointestinal tract disease. These
include the ability to adhere to human intestinal mucosa, the
provision of temporary and potentially permanen 1000 t colonisation
of the gastrointestinal tract, the production of antimicrobial
agents resulting in inhibition of pathogen growth, as well as
the tolerance to acid and bile. In addition LAB and fermented
milk products may possess anti-mutagenic and anti-carcinogenic
properties.10 Probiotic bacteria containing these properties
are known to exist and include a number of Lactobacillus
species.
Lactic acid bacteria produce a number of major fermentation products
including lactic acid, acetic acid, as well as hydrogen peroxide,
bacteriocins and other metabolites as described by Mishra and
Lambert11. Recent studies have shown that Lactobacillus
acidophilus and other lactic acid bacteria will inhibit
the growth of H. pylori in vitro12,13.
Lactic acid, acetic and hydrochloric acid inhibit H. pylori
growth in vitro (Fig 1). The concentrations of lactic acid produced by strains of LAB
tested ranged from 50 to 156 mmol/l and correlated with H.
pylori inhibition (Table 2). Six strains of Lactobacillus acidophilus and
one strain of Lactobacillus casei subsp and rhamnosus
inhibited H. pylori growth where as Bifidobacterium
bifidus, Pediococcus pentosaceus and Lactobacillus
bulgaricus did not13.
Other components produced by LAB may have in
vitro anti-Helicobacter properties. Included in these are
bacteriocins and antibiotic like substances14,15.
The bacteriocin nisin, potentiated by EDTA or citrate has in
vitro activity against H. pylori in vitro15.
Recent reports have reviewed the activities
of other milk components including lactoferrin. Probiotics may
be given in milk based products, whey, proteins and casei.16,17
Lactoferrin is a glycoprotein found in mammalian milk and is
known to possess activity against a variety of gram negative
bacteria. When tested in vitro against Helicobacter
pylori at concentrations up to 2 mg/ml inhibition was noted16.
A peptic digest of lactoferrin appeared to be relatively inactive.
Other components of milk protein which maybe administered along
with probiotics have been found to exert anti-Helicobacter effect
in vitro17.
|
Table 2.
Inhibition of Helicobacter pylori
NCTC 11637 by probiotic culture supernatant fluid in an agar well
diffusion assay
| Organism |
CSCC No.*
|
pH
|
L-Lactic acid (mmol/ l° )
|
H. pylori NCTC 11637**
|
1000
| Lactobacillus
acidophilus |
2400
|
3.9
|
70
|
0 ± 0
|
| L. acidophilus |
2401
|
3.9
|
95
|
1.5 ± 0.5
|
| L. acidophilus |
2403
|
3.9
|
133
|
2.0 ± 0.6
|
| L. acidophilus |
2404
|
4.0
|
91
|
2.5 ± 0.6
|
| L. acidophilus |
2405
|
4.1
|
87
|
2.1 ± 0.5
|
| L. acidophilus |
2406
|
4.0
|
86
|
1.1 ± 0.4
|
| L. acidophilus |
2409
|
4.1
|
85
|
0 ± 0
|
| L. acidophilus |
2422
|
4.1
|
121
|
1.9 ± 0.5
|
| L. casei |
2622
|
3.8
|
156
|
2.1± 0.4
|
| L. bulgaricus |
2515
|
3.8
|
50
|
0 ± 0
|
| Pediococcus
pentosaceus |
2304
|
4.9
|
37
|
0 ± 0
|
| Bifidobacterium
bifidus |
1900
|
5.6
|
12
|
0 ± 0
|
| 3% lactic acid |
|
2.3
|
445
|
3.5 ± 0.2
|
*CSCC = CSIRO Starter Culture Collection **
Average annular radius of inhibition of nine assays ± S.D. (mm)
***Modified from reference 13
|
| Figure 1.
Effect of acid type on inhibition of
H. pylori NCTC 11637 by agar well diffusion assay.
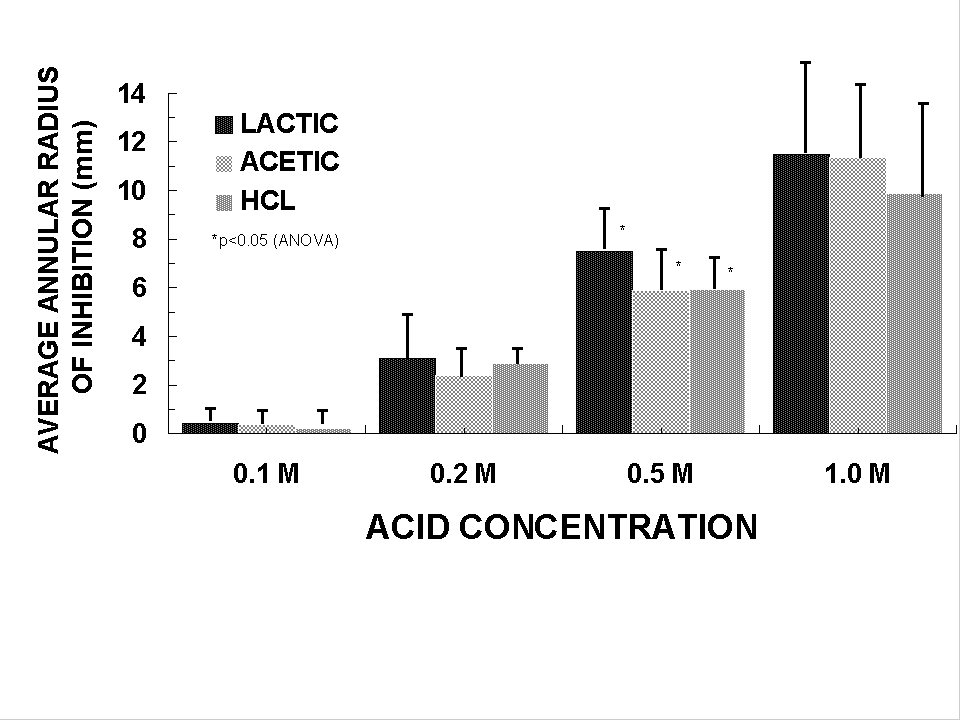
|
A pH study by Michetti and colleagues18,
has recently been undertaken in a randomised, double-blind, controlled
clinical trial incorporating a whey-based Lactobacillus acidophilus
(strain LA1) culture supernatant along with either a potent acid suppressor
in the form of omeprazole (with anti-H. pylori properties)
or placebo. Twenty volunteers were randomised in this study and treated
for a 14 day period. A breath test assessing H. pylori status
revealed a significant fall in the H. pylori colonisation following
the LA1 culture supernatant therapy. Of interest is the finding that
this effect was sustained over a period of 6 weeks post-treatment.
This study suggests that culture supernatant of a Lactobacillus is
a potential useful adjuvant for H. pylori treatment.
Other components of milk may also be useful in protection
of the gastric mucosa against different ulcerogenic agents. Milk phospholipids
may protect the animal19 and human20 gastric
mucosa against damage by exogenous ulcerogenic agents. Milk also has
been shown to contain substantial amounts of prostaglandins which
in animal experiments also protect against stress-induced gastric
ulceration.
Thus, there is accumulating evidence that a number
of LAB have in vitro and in vivo activity against
H. pylori infec 1000 tion. Moreover the effect of LAB in the prevention
of antibiotic induced diarrhoea when given as an adjunct to therapy
for H. pylori may be important. Currently up to 15% of subjects
develop side effects associated with antimicrobial therapy used to
treat H. pylori . Fermented milk products in the form of yoghurts
may also have additional potential benefits in suppressing H. pylori
as demonstrated in vitro.
Gastric malignancy is caused by H. pylori and
other forms of gastritis and a number of environmental factors including
smoking, vitamin C deficiency and mutagen and N-nitroso compound formation
from food10. Fresh vegetables, dairy foods, vitamin C,
vitamin A, carotene and selenium protect against the development of
cancer21. The role of milk based probiotic agents in the
development of malignancy has however not been reviewed as an independent
preventive factor. The theoretical benefits of LAB in the breakdown
of chemical carcinogens22 in decreasing undesirable bacterial
enzymes (including nitroreductase) implicated in carcinogenesis, as
well as altering the gastric mucosal permeability and structure are
potential mechanisms of action for prevention of malignancy. Antimutagenic
properties of milk fermented with Lactobacillus bulgaricus
and Streptococcus thermophilus have been demonstrated by Bodana
and Rao23. In addition, strains of lactobacilli and
E. coli have been shown to be active in degrading nitrosamines
suggesting a potential role of intestinal flora in degrading gastric
procarcinogens24.
Healthy human volunteers fed Lactobacillus
acidophilus strains NCFM and N-2 had a significant decrease
in the activity of three luminal bacterial enzymes- B glucuronidase,
nitroreductase and azoreductase25. These enzymes may release
carcinogens into the stomach and intestine. In spite of the in
vitro and in vivo data no long term preventive studies
evaluating fermented dairy products containing LAB have been conducted.
Summary and
conclusions
In summary, evidence is accumulating that lactic acid
bacteria may have some role in the management of upper gastrointestinal
tract disease. The anti-Helicobacter effects of these bacteria
as well as milk components, as demonstrated in vitro, lends
support for further evaluation of these agents. Moreover, the benefits
in prevention of side effects from antimicrobial agents when used
to eradicate this infection may be important.
The management of infections of the oropharynx and
oesophagus in subjects immuno-compromised requires aggressive treatment
often including toxic antiviral and antifungal agents. Concomitant
administration to both prevent and as adjunctive therapy for established
infection using probiotics may be of potential benefit in the future.
Although cancer of the stomach is common in certain
geographic areas of the world, the role of lactic acid bacteria and
probiotic organisms is unclear. The theoretical benefits of a regular
intake of probiotics including a decrease in pathogenic bacteria,
including Helicobacter, alterations in immune function, and
a decrease of potential carcinogens, are now becoming clearer. Further
evaluation in longitudinal studies of probiotics, particularly in
milk based products, are required to define their beneficial effects.
Chinese abstract
References
- Franklin MA, Skoryna SC. Studies on natural gastric
flora: Bacterial flora of fasting human subjects. Canadian Medica
1000 l Association Journal 1966; 95: 1349-1355.
- Drasar BS, Shiner M, McLeod GM. Studies on the
intestinal flora I. The bacterial flora of the gastrointestinal
tract in health and achlorhydric persons. Gastroenterology. 1969;
56: 71-79.
- Milton-Thomson GJ, Lightfoot NF, Ahmed Z et al.
Intragastric acidity, bacteria, nitrite and N-nitroso compounds
before, during and after cimetidine treatment. Lancet 1982; 2: 1091-1095.
- Mizurani. The relationship between micro-organisms
and the physiology of ageing. In Functions of Fermented Milk eds
Y Nakazawa, A Hosono. London: Elsevier Applied Science. 1992: 305-324
- Yeomans ND, Lambert JR. Infections of the Stomach
and Duodenum. In Bockus Gastroenterology 5th Edition. Eds Hanbrich
WS, Schattner F, Berk J E. Saunders London.1995: 805-815.
- Gray JPA, Shiner M. Influence of gastric pH on
gastric and jejunal flora. Gut 1967; 8: 574-581.
- Muscroft TJ, Deane SA, Youngs D et al. The microflora
of the post-operative stomach. Brit Journal of Surgery 1981; 68:
560-564.
- Berada N, Lemeland JF, Laroche G et al. Bifidobacterium
from fermented milk survival during gastric transit. J. Dairy Sci.
1991; 74: 409-413.
- Lambert JR, Lin SK, Aranda-Michel J. Helicobacter
pylori. Scand J of Gastroenterology 1995; 30 suppl 208: 33-46.
- Elder JB. Carcinoma of the Stomach. In Bockus Gastroenterology
5th Edition. Eds Haubrich WS, Shattner F, Berk JE. Saunders London.
1995: 805-815
- Mishra C, Lambert J. Production of anti-microbial
substances by probiotics. Asia Pacific J Clin Nutr 1996; 5(1): 20-24
- Bhatia SJ, Kochat N, Abraham P et al. Lactobacillus
acidophilus inhibits growth of Campylobacter pylori in vitro.
J Clin Micro 1989; 27; 2328-2330.
- Midolo PD, Lambert JR, Hull R, et al. In vitro
inhibition of Helicobacter pylori NCTC by organic acids and
lactic acid bacteria. J. Appl. Bacteriology 1995; 79: 475-479.
- Luo F, Lambert JR, Hull RR, Midolo PD. Anti-Helicobacter
factors produced by lactic acid bacteria. Amer J of Gastroenterology.
1998; 89: 1395.
- Projan SJ, Blackburn P. The bacteriocin nisin activated
by chelating agents is bactericidal for Helicobacter pylori
in vitro. Gastroenterology 1993; 104: 173.
- Dial EJ, Serna JH, Lichtenberger LM. Lactoferrin
inhibits the growth of Helicobacter in vitro. Gastroenterology
1995; 108: 82.
- Luo F, Hull RR, Lambert JR. Milk-derived substances
inhibitory to Helicobacter pylori in vitro. Amer J of Gastroenterology
1994; 89: 1395.
- Michetti P, Dorta G, Brassard D, Vouillamoz D et
al. Lactobacillus acidophilus supernatant as an adjuvant
in the therapy of Helicobacter pylori in humans. Gastroenterology
1995; 108: 166.
- Kiviluoto T, Paimela H, Mustonen H, Kivilaakso
K. Exogenous surface active phospholipid proteins. Necturus gastric
mucosa against luminal acid and barrier breaking agents. Gastroenterology
1991; 100: 38-46.
- Kivinen A, Tarpila S, Salminen S, Vapaatalo H.
Gastroprotection with milk phospholipids: a 786 first human study.
Milchwissenschaft 1992; 47: 694-696.
- Hirayama T. Methods and results of gastric cancer
screening. In Fielding JWL, Newmans CE, Ford CHJ eds. Gastric Cancer
Oxford Perganon Press; 1981: page 77-84.
- Bondana AR, Rao DR. Antimutagenic Activity of Milk
Fermented by Streptococcus thermophilus and Lactobacillus
bulgaricus. J Dairy Sci. 1990: 73; 3379-3384.
- Ayebo AD. Antitumor Components (s) of Yogurt: Fractionation.
J. Dairy Sci. 1981; 64: 2318-2323.
- Rowland IR, Grasso P. Degradation of N-Nitrosamines
by Intestinal Bacteria. Appl Microbiol 1975; 29: 7-12.
- Goldin BR, Gorbach SL. The effect of milk and lactobacillus
feeding on human intestinal bacterial enzyme activity. Amer J Clin
Nutr 1984; 39: 756-761.

Copyright © 1996 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
0