|
|
Asia Pacific J Clin Nutr (1995) 4: 304-313
Asia Pacific J Clin Nutr (1995) 4: 304-313

Review article
Diet, hyperlipidaemia and cardiovascular
disease
Jonathan M Hodgson BAgrSc(La Trobe), PhD(Monash), Mark L Wahlqvist BMedSci, MD(Adelaide) MD(Uppsala),FRACP,FAIFST,FACN,FAFPHM, Bridget Hsu-Hage BSc(Chung-Hsing),
MS(Columbia), PhD (Monash).
Reviewed here are results of intervention studies
examining relationships between diet and hyperlipidaemia, or diet
and cardiovascular disease (CVD). A reduction in the intake of saturated
fatty acids (SFAs) and trans-fatty acids (TFAs), and an increase
in the intake of polyunsaturated fatty acids (PUFAs), are favourable
to lipoprotein status. Where a reduction in total fat intake is
achieved by a reduction in dietary SFAs, there would appear to be
a favourable effect on CVD events and mortality, although the evidence
for this from intervention studies is not strong. Adequate dietary
PUFA intake, both w 6 and w 3, may be associated with
reduced risk for CVD events more via pathways other than those which
operate through lipoproteins. Other macronutrients including carbohydrates,
proteins and alcohol can have significant effects on lipoproteins,
although the effects of dietary intervention with these nutrients
on coronary and total mortality are virtually unknown. Non-nutrient
components of foods with small lipid lowering properties may be
cumulatively important in an overall diet. In relation to food,
results of secondary intervention studies provide support for a
beneficial role of plant food and fish in reducing coronary and
total mortality. Therefore as far as both hyperlipidaemia and CVD
are concerned, the total dietary approach may be more important
than the single nutrient approach.
Introduction
Dietary modification of serum lipoproteins is usually
intended to reduce cardiovascular and total mortality. Available studies
deal with the way diet changes serum lipoproteins, as well as atherosclerosis,
coronary artery disease (CAD), myocardial infarction (MI), and mortality.
Dietary management of hyperlipidaemia therefore concerns not only
the modification of serum lipoproteins, but more importantly the prevention
of cardiovascular disease (CVD) and mortality.
The majority of studies which have examined relationships
between diet and CVD or CVD risk factors have emphasised the fat component
of the diet. Other dietary components, including protein, carbohydrate,
vitamins, minerals and non-nutrient components of food may affect
serum lipoproteins and CVD risk. However, in most populations the
quantity and quality of fat in the diet is believed to be a powerful
dietary indicator of CVD risk.
The evidence reviewed here includes 1000 intervention
studies which have examined the relationship between diet and serum
lipids and lipoproteins, as well as CVD end points. Where information
from intervention studies is limited, evidence from prospective, cross-sectional
and case-control studies is examined.
Diet and cardiovascular disease: an introduction
The "diet-heart" hypothesis was proposed
to explain the relationship between diet, fatty acids in particular,
and CVD. According to this hypothesis, a high intake of saturated
fatty acids (SFAs) and cholesterol and a low intake of polyunsaturated
fatty acids (PUFAs) increases serum cholesterol, which leads to the
development of atheromatous plaques in the coronary arteries. Accumulation
of these plaques leads to narrowing of the coronary arteries, reduced
blood flow to the heart muscle, and finally to the occurrence of MI1.
This hypothesis is supported by two lines of evidence: studies which
have associated dietary fatty acids with serum cholesterol concentrations,
and studies which have found a positive association between serum
cholesterol concentration and CVD. Various other dyslipidaemias, including
low high-density lipoprotein (HDL) cholesterol concentration, raised
serum triglyceride concentration, increased serum concentration of
small dense low-density lipoprotein (LDL) particles, and poor chylomicron
remnant clearance, have now been associated with increased risk of
CVD. Dietary fatty acids have also been related to CVD risk through
these other dyslipidaemias. These developments have altered the hypothesis
to include many other aspects of lipid and lipoprotein metabolism.
The key role which thrombosis plays in CVD was not
included in the "diet-heart" hypothesis, and until recently
thrombosis was regarded separately to the process of atherosclerosis.
The development of coronary thrombosis as a result of fissuring of
an atherosclerotic plaque is the major determinant of progression
of stable atherosclerotic lesions to MI2. Thrombosis may
also be involved in atherosclerotic plaque development. Atherosclerotic
plaques may increase in size after rupturing and thrombus formation3.
Furthermore, lipoproteins might influence thrombosis via effects on
coagulation, in addition to their role in atherosclerosis4.
Dietary fatty acids have also been linked to thrombosis3.
Omega-3 fatty acids in particular have been shown to be antithrombotic.
However, there is no clearly established prothrombotic effect of saturated
fatty acids, although this has not been ruled out5.
The role of diet in CVD has been summarised briefly
in Figure 1. Blood factors, including lipoproteins and platelet function,
and arterial wall function, with its effects on coagulation, blood
pressure, and organ perfusion influence the processes of arteriosclerosis,
atherosclerosis and thrombosis, which can lead to CAD. Coronary artery
disease, along with these other processes may result in angina, MI
or death. Diet may also influence CVD through pathways other than
atherosclerosis and thrombosis6. For example CVD may result
from poor myocardial function. Particular micronutrients, including
selenium deficiency7 and cobalt toxicity8 may
lead to cardiomyopathy, impaired myocardial function, and congestive
heart failure. There is also evidence that myocardial function can
be influenced by dietary fat9,10, and alcohol11.
| Figure 1.
Potential pathways linking diet to cardiovascular disease. |
 |
In re 1000 lation to CVD, most intervention studies
have investigated links between diet and hyperlipidaemia, hyperlipidaemia
and CVD end points, or diet and CVD end points presumed to be operating
through hyperlipidaemias. Outlined in Figure 1 are several other pathways
through which diet might influence CVD. The importance of these pathways
to CVD is recognised, and it is recognised that many of the changes
in CVD produced by dietary intervention may relate to pathways other
than those which operate through serum lipids and lipoproteins. The
main focus in this review, however, is the diet-hyperlipidaemia-CVD
pathway.
The nature of diet
Diet or food intake may be described, or changed,
in terms of foods or nutrients. Most dietary intervention studies
have examined one particular nutrient, namely fat, but other dietary
components including protein, carbohydrates, dietary fibre and alcohol
have also been studied. Few studies have used foods such as plant-derived
or fish as the dietary intervention. Even here, the assumption in
studies of food intervention is that specific nutrient effects are
usually in question. However, foods contain more than one nutrient,
and indeed many non-nutrients of biological importance. Some of the
effects observed may therefore be due to other factors in food, although
the evidence for certain nutrient relationships is quite strong. Secondary
dietary changes resulting from the desired intervention should also
be taken into account. For example, a reduction in the total fat intake
will usually result in changes in carbohydrate and/ or protein intake.
Predicting dietary responsiveness
Not all individuals will respond to the same dietary
change with the same change in serum lipoprotein status, let alone
in cardiovascular event or total mortality end points. There are several
reasons for these differences which need to be taken into account
in the evaluation of intervention studies. The background diet of
the study community or individual is one of the most important considerations.
In addition, there are genetic determinants of hyperlipidaemia or
atherosclerosis susceptibility. These include familial hypercholesterolaemia
which is usually poorly responsive to diet, although ordinarily LDL
receptors are responsive to dietary change12. Apo E status
is indicative of responsiveness of serum lipids to dietary fat change,
with apo E4 being more responsive than apo E313,14.
There are also non-dietary lifestyle factors such as physical inactivity
and cigarette smoking which may influence dietary responsiveness.
Energy balance and hyperlipidaemia
Increased body fatness represents positive energy
balance, whether for reasons of excessive intake or under-expenditure.
Over fatness and an abdominal distribution of fatness are the most
potent factors in increasing VLDL triglyceride and LDL cholesterol
and decreasing HDL cholesterol15. An increasing prevalence
of obesity (body mass index >30 kg/ m2) in the Australian
population during the 1980s is therefore of considerable importance16.
Dietary fat and hyperlipidaemia
Introduction
Dietary fat may be derived from animal or plant sources.
The most abundant type of dietary fat is triglyceride, which may provide
SFA, monounsaturated fatty acids (MUFA), and PUFA. Most PUFAs in the
diet are essential fatty acids (EFA) or EFA derivatives. There are
two classes of EFAs, omega-6 (w 6) and omega-3 (w 3). Linoleic acid (18:2w 6) and 1000 a -linolenic acid (18:3w 3) are the precursor or parent w 6 and w 3 EFAs, from which longer chain EFAs are
derived by enzyme desaturation and elongation. The same group of enzymes
are shared between fatty acid classes (Fig. 2). SFAs and MUFAs are
not essential, because humans possess the ability to derive these
from protein and carbohydrate if necessary. Although trans-fatty
acids (TFA) can be classed as either MUFA or PUFA, they are often
classified as a separate group because most unsaturated fat, both
dietary and in vivo derived, in humans is in the cis
configuration. The fatty acid classes can also be described in terms
of individual fatty acids. This is useful when different fatty acids
within one class have different metabolic effects. Cholesterol and
phospholipids are also important dietary fats.
Hyperlipidaemia has been classified as type IIA (raised
LDL cholesterol), type IIB (raised LDL cholesterol and raised VLDL,
characterised by an elevated fasting serum triglyceride measurement),
or type IV (raised VLDL only). More recently, the term dyslipidaemia
has been used to describe hyperlipidaemias as well as low HDL cholesterol
concentration, raised serum triglyceride concentration, increased
serum concentration of small dense LDL particles in serum, and poor
chylomicron remnant clearance.
| Figure 2.
Metabolic pathways for the conversion of w 6 and w 3 essential fatty acids
to essential fatty acid derivatives. |
 |
Fatty acid classes
In one of the earliest studies on dietary fat and
serum cholesterol, Kinsell et al17 found that diets high
in vegetable fat lowered serum cholesterol concentrations, findings
that were confirmed in the same decade18-23. These studies
established that serum cholesterol concentrations were more upwardly
responsive to dietary saturated fat than to total fat or cholesterol
in the diet. After comparisons of different fats and oils in these
studies, it was proposed that SFAs were responsible for a hypercholesterolaemic
effect, and that PUFAs were responsible for a hypocholesterolaemic
effect18,21,23. Formulae to predict the expected change
in serum cholesterol with changes in SFAs, PUFAs, and cholesterol
were developed separately by Keys et al 22,24,25 and Hegsted
et al 26,27. A recent meta-analysis of 27 trials28,
on the effects of dietary fatty acids on serum lipids and lipoproteins
produced an equation which was in close agreement with those of Keys
et al and Hegsted et al.
These studies showed that serum cholesterol is much
more responsive to changes in dietary SFAs than either dietary PUFAs
or cholesterol. In the studies by Hegsted et al26 the changes
in SFAs accounted for over 70% of the variations in serum cholesterol.
Dietary cholesterol has a significant, although minor contribution
to serum cholesterol changes. Although both the equations of Keys
et al25 and Hegsted27 were able to predict well
the effects of changes in dietary fat on serum cholesterol, several
investigators have found large individual variability in response
to changes in dietary SFAs, PUFAs24,29, and especially
to dietary cholesterol< 1000 sup>30,31.
The question of the appropriate ratio of PUFAs to
SFAs (P:S ratio) has been addressed by Gustafsson et al32,33.
In these studies, diets with different P:S ratios were compared with
respect to serum lipoprotein changes. It was found that increasing
the P:S ratio above 0.7 did not improve serum lipoproteins in patients
with moderate hyperlipidaemia. Most of the benefits in relation to
the lipoproteins were therefore gained with a shift in the P:S ratio
up to 0.7. However, this level might depend upon factors affecting
dietary responsiveness.
There is also evidence in support of the relationship
between increasing the P:S ratio and improving serum lipoproteins
in a large scale study. In the Lipid Research Clinics Primary Prevention
Trial, in which over 6000 hypercholesterolaemic men were advised to
adopt a diet lower in SFAs and cholesterol and relatively higher in
PUFAs, SFAs were directly related and PUFAs were inversely related
to LDL cholesterol lowering34.
Apart from the specific fatty acid composition of
the diet, several other dietary factors are important determinants
of effects on serum lipids and lipoproteins. The most important of
these is probably the background diet. The prevailing level of SFAs
in the diet, for example, might influence the degree to which a reduction
of SFAs will reduce LDL cholesterol. In addition, the structure of
the triglyceride in the fat being consumed can also influence serum
lipid and lipoprotein responses35,36.
Saturated fatty acids
Myristic acid (C14:0) has the greatest cholesterol
elevating effects25,26 and has been estimated to be four
to six times as cholesterol raising as either lauric (Cl2:0) or palmitic
(C16:0) acid37. However, the effects of individual fatty
acids on serum cholesterol can still only be estimated. Refinement
of the Keys and Hegsted equations is proceeding with more metabolic
studies. The more recent studies indicate that a change in palmitic
acid intake has relatively little effect on LDL cholesterol concentration,
particularly when compared to change in myristic acid ingestion38-40.
A number of experiments have indicated that stearic acid (C18:0) does
not elevate cholesterol25,26,41. Saturated fatty acids
of chain length less than C10 produce little or no cholesterol elevation
25,42,43. These results are summarised in Figure 3.
| Figure 3
Response of serum total cholesterol to changes in fatty acid intake.
|
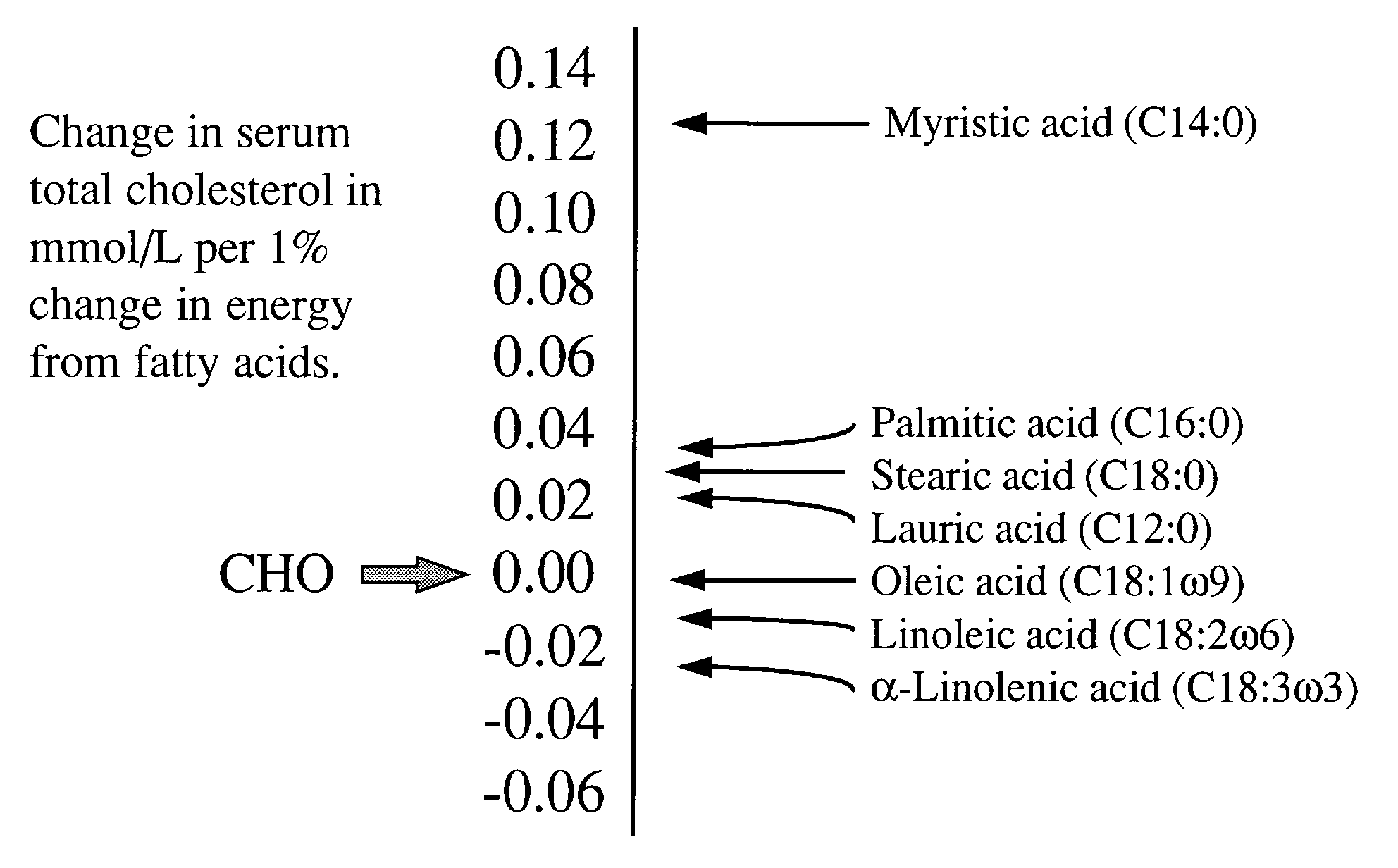
Estimates taken from meta-analysis of Mensink
and Katan (28).
Figure adapted from Grundy (44). |
Monounsaturated fatty acids (MUFAs)
The major MUFA in the diet is oleic acid (18:1 n-9).
It is widespread in the food supply, the richest sources being olive
oil and canola oil. The effects of MUFAs on serum cholesterol have
been examined in a number of studies45-47, and it was found
in all of these studies that MUFAs did not elevate serum cholesterol
concentrations as did SFAs, and that diets high in MUFAs did not lower
HDL cholesterol concentrations, as did substitution of SFAs with carbohydrates.
It has been estimated from a meta-analysis28 that if monounsaturated
fatty acids replace carbohydrate in the diet then a relatively small
decrease in LDL cholesterol and a small increase in HDL cholesterol
is expected.
Polyunsaturated fatty acids (PUFAs)
1000
Although increasing linoleic acid will lower LDL cholesterol,
its effects are less than half that of lowering dietary saturated
fatty acids28. The serum cholesterol lowering potential
of linoleic acid is shown in Figure 3.
Fish and fish oils are major sources of omega-3 (w 3) fatty acids. Fish oils consistently reduce serum triglyceride concentrations,
particularly in hypertriglyceridaemic subjects48-50. Although
substitution of SFAs with long chain w 3 PUFAs has been found to result in a fall
in total cholesterol19,21 and LDL cholesterol, this effect
seems to be due to reduced SFAs. Supplementation with
w 3 PUFAs has also been reported not to lower LDL cholesterol51,
and lowering of serum triglycerides with fish oils is often associated
with an increase in LDL cholesterol52, particularly in
association with diabetes53. However, different w 3 PUFAs may have different effects on serum
cholesterol concentrations.
Fish oils also affect the haemostatic system and eicosanoid
metabolism. The overall result of an increase in the intake of
w 3 fatty acids is a beneficial change in the haemostatic balance towards
a more vasodilatory state, with reduced platelet aggregation. There
is evidence that much of the proposed beneficial effects of the
w 3 fatty acids on cardiovascular disease may operate through the haemostatic
system and eicosanoid metabolism rather than through lipoproteins,
thereby reducing the risk of MI through influencing thrombosis.
Trans-fatty acids (TFAs)
Many vegetable oils require partial hydrogenation
to attain properties needed for particular food uses. This process
generates a variety of trans and uncommon cis-fatty
acid isomers. The other major source of TFAs is from ruminant animal
origins.
A number of studies have examined the relationship
between TFAs in the diet and serum lipids and lipoprotein concentrations.
Many of these studies, conducted during the 1960s, produced inconsistent
findings. More recently, well-designed studies have found that TFAs
increase LDL cholesterol54,55 and Lp(a)38,56,
and reduce HDL choles-terol54,55, and overall are approximately
as unfavourable on serum lipoproteins as SFAs in general. It is not
known whether particular TFAs are responsible for the observed effects.
Modification of other macronutrient intakes and
hyperlipidaemia
Carbohydrates
The effects of dietary fatty acids on serum lipids
and lipoproteins are often measured in relation to carbohydrate intakes,
which are assumed to be neutral in these analyses. However, high carbohydrate
diets reduce LDL cholesterol46, although their beneficial
effects seem to be secondary to a reduction in dietary SFAs. High
carbohydrate diets may also be associated with increased VLDL production
and elevated triglyceride levels, and falls in HDL cholesterol28,57.
It must be kept in mind however, that serum lipoproteins are not the
most important outcome. Cardiovascular disease and death are obviously
more important.
Protein
There is some evidence which suggests that the source
of protein (animal vs plant) has differential effects on serum lipoproteins.
Soy protein based diets have been shown to lower serum LDL cholesterol
in hyperlipidae 1000 mic subjects58-61. However, the effect
is less consistent in normocholesterolaemic people62-64.
Alcohol
Alcohol consumption produces an increase in serum
triglyceride concentrations as a result of elevation of VLDL and chylomicron
levels65. There is also some elevation of serum cholesterol
levels. A proportion of this is due to an increase in HDL cholesterol66,67.
The rise in HDL cholesterol occurs only in inactive individuals, not
in runners where HDL levels are already raised68.
There is also some evidence for an inverse association
between alcohol intake and LDL cholesterol. In the Lipid Research
Clinics Coronary Primary Prevention Trial, change in alcohol intake
was associated inversely with change in LDL cholesterol levels among
men in the placebo group after adjustment for body mass index and
dietary lipids69.
Fibre
Numerous studies have suggested that an increased
consumption of fibre-rich foods can reduce serum cholesterol levels70-72.
However, not all dietary fibre appears to influence serum lipoproteins.
Insoluble fibre (such as wheat bran) has little influence on serum
lipoproteins73. Soluble fibres appear to favourably affect
serum lipoproteins. However, the effects are variable depending on
the type of soluble fibre used. For example, guar gums tend to lower
LDL cholesterol, but not influence HDL cholesterol, whereas oat bran
will lower LDL cholesterol as well as increase HDL cholesterol74.
Oat bran may also lower triglycerides in hypercholesterolaemic people75.
Micronutrients
Niacin in doses used to lower serum cholesterol should
be regarded as a pharmacological rather than a nutritional approach.
There is little evidence that other micronutrients can influence serum
lipid and lipoprotein concentrations. However, there may be several
micronutrients which influence atherosclerosis via effects on lipoproteins
without significantly altering lipoprotein concentrations76.
Non-nutrient food components and hyperlipidaemia
There is growing interest in various non-nutrient
components of food which favourably influence plasma lipoprotein status.
At the moment, these identified components should be regarded as indicative
of new ways of looking at food from the point of view of the management
of hyperlipidaemia. The components include:
- a lipid soluble fraction from boiled coffee77,
- allicin from garlic78,79,
- saponins from foods like chick peas80,
- tocotrienols from barley and palm oil, which appear
to have HMG CoA reductase inhibitor activity81,82
- and plant sterols which may be handled alternatively
to cholesterol83.
With the growing evidence for physiological effects
of phytoestrogens in humans84 and serum cholesterol-lowering
properties in experimental animals of certain natural food colours
like anthocyanins85,86, there may be an ever wider range
of foods of value in the management of lipid disorders. Although the
effects of individual food components may be relatively small (say
a 1-3% lowering of LDL cholesterol) cumulatively, several components
could be important.
Diet and cardiovascular disease
Cardiovascular end points which have been used in
dietary intervention studies include CAD, MI, CVD mortality, and total
mortal 1000 ity.
Coronary artery disease
Coronary angiography has been used to assess CAD progression
or regression in humans in several studies. However, detailed analysis
of nutritional variables, including fatty acids, has only been performed
in two quantitative angiographic studies87,88.
The influence of diet on the appearance of new lesions
in human coronary arteries was examined in the placebo arm of the
Cholesterol Lowering Atherosclerosis Study (CLAS) study by Blankenhorn
et al89. Coronary angiograms along with 24-hour dietary
recall information were used to examine the relationships between
change in diet and the appearance of new lesions. The placebo group
was given dietary goals: to reduce total fat to less than 26% (5%
SFAs, 10% MUFAs and 10% PUFAs). It was found that increased intake
of total fat, PUFAs, linoleic acid (18:2w 6), oleic acid (18:1w 9), and lauric acid (12:0),
was associated with a significant increase in risk of new lesions.
The results in this study indicated that when total dietary fat and
SFAs are reduced, the preferred substitutes may be protein and carbohydrate
rather than PUFAs and MUFAs89.
More comprehensive dietary data was collected in the
St Thomas’ Atherosclerosis Regression Study (STARS)90.
Dietary assessments were performed using a dietary history method
on all patients at least twice during the study. Pooled data from
the usual care and lipid lowering diet groups were used to assess
the relationships between nutrient intake and CAD. Total fat and SFA
were the nutrients most closely (positively) associated with CAD progression.
MUFAs were also positively associated with CAD progression, but this
may have been due to a close relationship with total and SFA intake.
PUFAs were not significantly related to CAD progression.
Other angiographic trials with dietary interventions,
with or without additional interventions91-93, are in general
agreement with the CLAS89 and STARS90, and indicate
that lower total and saturated fat intakes may result in reduced progression,
or regression of CAD. The results are also consistent with an effect
of SFA intake on atherosclerosis operating through serum lipoproteins.
The relationships of MUFAs and PUFAs with CAD progression is less
clear. The lack of a negative relationship between PUFAs and CAD progression
in the STARS90, and the positive relationship between both
PUFA and linoleic acid intake and CAD in the CLAS89 suggests
that SFAs may be more important in relation to CAD. The results of
the CLAS89 are consistent with results from a recent cross-sectional
study where a positive relationship between linoleic acid and CAD
was found94. The results from both the STARS90
and the CLAS89 are at variance with data finding negative
relationships between linoleic acid and CVD events95-97.
These varying results may reflect a beneficial influence of dietary
PUFAs, including linoleic acid, on processes other than atherosclerosis
which influence CVD events.
Cardiovascular disease events and mortality
Primary intervention trials
Studies of diet as the only intervention, aiming for
a reduction of cardiovascular mortality and/ or CVD incidence are
presented in Table 1. Few primary intervention trials have included
changes in diet as the only intervention98-100. In the
study by Dayton et al98, the effects were examined of two
diets containing about 40% of energy from fat, but with less SFAs
and more PUFAs in the experimental diet than the control 1000 diet.
The experimental diet, which contained 35 to 40% of total fat intake,
each of linoleic and oleic acid, reduced serum cholesterol by 12.7%.
The experimental diet was associated with a 31% reduction in all atherosclerosis
related events There was little difference in total mortality rates,
however.
Table 1. Primary prevention trials of dietary
intervention aiming for a reduction in cardiovascular mortality or
incidence.
| Study/ Author |
Randomised
|
Study Population
|
Diet
|
Cholesterol Reduction
|
Major Findings
|
| Los Angeles Veterans
Administration Study Dayton et al 1969 |
Yes
|
846 men aged 55 to 89
|
High P:S ratio
|
13% }
(7 years).
|
31% reduction in all cardiovascular events No reduction in total
mortality
|
| Finnish Mental Hospital
Study Miettinen et al 1972 |
No
(Cross-over)
|
1900 men
|
High P/S ratio(1.42-1.78)
|
15%
(12 years).
|
Reduced mortality from CHD No reduction in total mortality
|
| Minnesota Coronary Survey
Frantz et al 1989 |
Yes
|
4393 men &
4664 women
|
High P:S ratio (0.28[control] c.f1.67[treatment])
|
15%
(1 year)
|
No significant reduction in CVD events, CVD mortality or total
mortality
|
Another of the diet-only primary intervention studies
was the Finnish Mental Hospital Study100. The mortality
from CHD and other causes was studied in a controlled 1000 trial with
cross-over design. In one hospital a cholesterol lowering diet was
introduced, with a PUFA to SFA ratio of 1.42 to 1.78, and in the other
hospital a usual diet, with PUFA to SFA ratio of 0.22 to 0.29, served
as the control. After six years, the diets were reversed and the trial
continued for a further six years. In men, the high PUFA diet was
associated with reduced mortality from CHD. Total mortality was also
lower on the experimental diet, but not significantly. For women,
the differences for both CHD mortality and total mortality were not
significant.
In a study by Frantz et al99, two diets
with similar total fat (39% [control] and 38% [treatment]) and MUFA
(16% and 18%) intakes, but with differing SFA (18% and 9%), PUFA (5%
and 15%) and cholesterol (446 mg and 166 mg) intakes, were compared
with respect to CVD events, CVD mortality and total mortality. No
differences were observed for any of the end points between the two
diets.
Other dietary intervention trials aiming for a reduction
in CVD incidence and/ or mortality have considered other CVD-risk
factors as well as dietary change, where the effect of dietary change
is often confounded with other factors.
Secondary intervention trials
Several secondary intervention trials have been conducted
(Table 2). In three of the most successful of these trials, in relation
to CVD events, CVD mortality and total mortality, the aim of the successful
intervention was to alter the intake of a particular food, foods or
diet in general101-103. Most of the studies which have
failed to show a reduction in events or mortality used an intervention
which focused on reducing total fat or increasing the P:S ratio104-107.
Table 2. Secondary prevention trials of dietary
intervention aiming for a reduction in cardiovascular mortality or
incidence.
| Study/ author |
Randomised
|
Study Population
|
Diet
|
Cholesterol Reduction
|
Major Findings
|
| Morrison114
1955 |
No
|
100 subjects aged 40-79 years
|
Low fat
|
29%
|
Reduced mortality
|
| Rose et al al 1965 |
Yes
|
52 subjects aged <70 years
|
Low fat added corn and olive oils
|
1000
Corn oil 20% Olive oil no change
|
No reduction in mortality between the groups
|
| MRC 1965 |
Yes
|
252 subjects aged <65 years
|
Low fat
|
8% (3 years)
|
No reduction in morbidity or mortality
|
| MRC 1968 |
Yes
|
393 subjects aged <60 years
|
High P:S ratio soya-bean oil(2.0)
|
17% (at 3 years)
|
Reduced relapse rate No reduction in cardiovascular mortality,
or total mortality
|
| Leren115
l970
|
Yes
|
412 subjects aged 30-64 years
|
High P/S ratio (2.4)
|
14% (5 years)
|
Reduced mortality due to myocardial infarction. No difference
in total mortality
|
| Bierenbaum116
et al 1973 |
No
(matched controls)
|
200 subjects aged 30-60 years
|
High P:S ratio. (2.6)
|
10% (10 years).
|
Reduced mortality from myocardial infarction. And reduced total
mortality
|
| Woodhill et al 1978 |
Yes
|
458 subjects aged 30-59 studied for 2-7 years
|
High P:S ratio (1.5)
|
Intervention. 11%. Controls 7%
|
No difference in mortality
|
| Burr et al 1989 |
Yes
|
2033 men studied for 2 years
|
Low fat, high fibre, or increased fish intake
|
|
29% reduction in all cause mortality in those on the increased
fish intake
|
| Singh et al 1992 |
Yes
|
406 subjects
|
Advice to eat fruits, nuts, vegetables, pulses, & fish
|
Intervention 13% Controls5 %
|
39% reduction in cardiac events, 45% reduction in total mortality
|
| de Logeril et al 1994
|
Yes
|
605 subjects
|
Advice to eat a "Mediterranean" diet, high in bread,
fruit, vegetables & fish; less red meat; butter & cream
replaced with high 18:3w 3 margarine
|
Intervention 5% Control 5%
|
Significant reduction in CVD deaths & total mortality
|
In a randomised controlled study by Burr et al101,
the effects of dietary intervention on secondary prevention of myocardial
infarction were examined. It was found that an increased intake of
fatty fish reduced 2 year all causes mortality by 29%. In another
secondary prevention study in patients with recent MI, CVD events
and total mortality were significantly reduced with dietary intervention103.
The dietary intervention which was associated with lower mortality
was advice to include more fruit, nuts, vegetables, grain products,
and fish in the diet. This advice was associated with significantly
lower SFA and MUFA intakes, and significantly higher PUFA intake,
as well as a significant reduction in weight. Other macronutrient
and micronutrient differences were also observed103. In
the study by de Logeril102, mortality was significantly
lower in an intervention group who were encouraged to adopt a "Mediterranean-type"
diet: more bread, root vegetables, green vegetables, fruit and fish;
less red meat; and with butter and cream to be replaced by a canola
oil based margarine high in a -linolenic aci 1000 d (C18:3w 3). After intervention, this group consumed significantly less fat,
SFAs, cholesterol, and linoleic acid, and more oleic and a -linolenic acid. The authors contributed much of the reduction in mortality
to the increased a -linolenic acid, however, other dietary
changes are likely to have contributed to the reduced mortality. The
mechanisms for the observed reductions in total mortality in the studies
by Burr et al101, Singh et al103 and de Logeril
et al102 may have been many and related to the effects
of w 3 fatty acids on blood factors, arterial
wall function and myocardial function (Fig. 1). Alterations in lipoproteins
and atherosclerosis may have been involved, but were probably less
important than other pathways.
Recently Truswell108 performed a meta-analysis
on dietary intervention studies and their effects on CVD events, CVD
mortality and total mortality. Although most have failed to show a
significant effect of intervention on CVD mortality or total mortality,
it was estimated from this analysis that the relative risk of death
from all causes was 0.94 (95% CI: 0.894-0.988), a significant reduction.
The intervention in these trials varied, and included low fat, altered
fat, increased fish, altered diet in general, smoking cessation or
exercise, or a combination of these. It is therefore difficult to
attribute the reduced mortality to specific dietary factors. However,
the results do suggest that dietary intervention can reduce total
mortality.
Foods and cardiovascular disease. prospective studies
Prospective studies have shown that many dietary interventions
can favourably influence serum lipid and lipoprotein concentrations.
Diets low in total and SFAs, and with sufficient
w 6 and w 3 PUFAs; relatively high in carbohydrate and protein; low in alcohol;
and with a variety of plant foods with various lipid lowering properties
will favourably modify most dyslipidaemias. Prospective studies also
show that people who have a higher energy intake109-112
indicative of greater physical activity, a high plant food intake109,
and a higher intake of fish113 have lower risk of CVD.
Conclusion
It is evident from intervention studies that diet
can influence hyperlipidaemia. A positive energy balance, characterised
by obesity and abdominal obesity, is one of the most powerful factors
in increasing serum LDL cholesterol and triglyceride concentrations,
and decreasing HDL cholesterol concentration. Of the macronutrients,
dietary fat has the most potent effect. A reduction in the intake
of SFAs and TFAs, and an increase in the intake of PUFAs, have favourable
effects on LDL and HDL cholesterol, and triglyceride concentrations.
Other macronutrients can also have significant effects on lipoproteins.
High carbohydrate diets reduce LDL cholesterol and HDL cholesterol,
and may increase triglyceride levels. Some of these effects may be
secondary to changes in dietary fat intake. It is still not clear
whether the type of protein in the diet can have significant effects
on serum cholesterol and triglyceride concentrations. Soluble fibres
appear to favourably affect serum LDL cholesterol, and some may increase
HDL cholesterol and lower triglycerides. Numerous non-nutrient components
of food have been identified as having minor lipid lowering properties.
Cumulatively, these may be important in the overall diet.
Diet has also been s 1000 hown to alter CVD risk.
The mechanisms involved may be many, and relate to factors other than
hyperlipidaemia. Where a reduction in total fat intake is achieved
by a reduction in dietary SFAs, there would appear to be a favourable
effect on CVD events and mortality, although the evidence for this
from intervention studies is not strong. The mechanisms implicated
here are probably related to the hyperlipidaemia-atherosclerosis link.
Higher dietary PUFA intake, of both
w 6 and w 3, may be associated with reduced risk for CVD events, perhaps more through
thrombosis and other processes than atherosclerosis. The effects of
dietary intervention with carbohydrates, protein, alcohol, fibre,
various micro-nutrients, or different non-nutrients, on coronary and
total mortality is virtually unknown. There is, however, growing evidence
that higher plant food intakes, and therefore carbohydrate intakes,
may favourably influence CVD. In relation to food, results of secondary
intervention studies provide support for a beneficial role of plant
food and fish in reducing coronary and total mortality. This view
is supported by results of prospective studies. Therefore as far as
both hyperlipidaemia and CVD are concerned, the total dietary approach
may be more important than the single nutrient approach.
Diet, hyperlipidaemia and cardiovascular
disease
Jonathan M Hodgson, Mark L Wahlqvist,
Bridget Hsu-Hage
Asia Pacific Journal of Clinical
Nutrition (1995) Volume 4, Number 3: 304-313

References:
- Willett W. Diet and coronary heart disease. In:
Willett W, editor. Nutritional epidemiology. Vol 15. New York: Oxford
University Press, 1990: 341-79.
- Eisenberg PR. Thrombosis and fibrinolysis in acute
myocardial infarction. Alcohol Clin Exp Res 1994;18:97-104.
- Nordoy A, Goodnight SH. Review. Dietary lipids
and thrombosis. Arteriosclerosis 1990;10:149-63.
- Miller W. Lipoproteins and the haemostatic system
in atherothrombotic disorders. Balliers Clin Haem 1994; 7: 713-32.
- Hoak JC. What is the historical background of research
on the role of fatty acids in thrombosis. Am J Clin Nutr 1992; 56:
786S.
- Wahlqvist ML. International trends in cardiovascular
diseases in relation to dietary fat intake: interpopulation studies.
In: Taylor TG, Jenkins NK, eds. Proceedings of the XIII international
congress of nutrition. London: John Libbey 1985: 539-43.
- Oster O, Prellwitz W. Selenium and cardiovascular
disease. Biol Trace Elem Res 1990; 24: 91-103.
- Jarvis JQ, Hammond E, Meier R, Robinson C. Cobalt
cardio-myopathy. A report of two cases from mineral assay laboratories
and a review of the literature. J Occup Med 1992; 34: 620-6.
- McLennan P. Relative effects of dietary saturated,
monounsaturated and polyunsaturated fatty acids on cardiac arrhythmias
in rats. Am J Clin Nutr 1993; 57: 207-12.
- Thuesen L, Nielsen TT, Thomassen A, Bagger JP,
Henningsen P. Beneficial effect of a low-fat low-calorie diet on
myocardial energy metabolism in patients with angina pectoris. Lancet
1984; 2: 59-62.
- 1000 Piano MR, Schwertz DW. Alcoholic heart disease:
a review. Heart Lung 1994; 23: 3-17.
- Goldstein JL, Brown MS. Familial Hypercholesterolemia.
In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown
MS eds. The metabolic basis of inherited disease 5th edn. New York:
McGraw -Hill, 1983: 672-712.
- Miettinen TA. Impact of apo E phenotype on the
regulation of cholesterol metabolism. Ann Med 1991; 23: 181-6
- Savolainen MJ, Pantala M, Kervinen K, Jarvi L,
Savanto K, Rankla T. Magnitude of dietary effects on plasma cholesterol
concentration: role of sex and apolipoprotein E phenotype. Atherosclerosis
1991; 86: 145-52
- Bjorntorp P, Smith UA. Distribution of body fat
and health outcome in man. Proc Nutr Soc Aust 1987; 12:11-22.
- Bennett SA, Magnus P. Trends in cardiovascular
risk factors in Australia: results from the National Heart Foundation's
Risk Factor Prevalence Study 1980-1989. Med J Aust 1994; 161: 519-27.
- Kinsell LW, Partridge J, Boling L, Margen S, Michaels
G. Dietary modification of serum cholesterol and phospholipid levels.
J Clin Endocrinol 1952; 12: 909-13.
- Ahrens EH Jr, Hirsch J, Insull W Jr, Tsaltas TT,
Blomstrand R, Peterson ML. The influence of dietary fats on serum-lipid
levels in man. Lancet 1957; 1: 943-53.
- Ahrens EH Jr, Insull W Jr, Hirsch J, et al. The
effect on human serum-lipids of a dietary fat, highly unsaturated,
but poor in essential fatty acids. Lancet 1959; 1: 115-9.
- Bronte-Stewart B, Keys A, Brock JF, Moodie AD,
Keys MH, Antonis A. Serum-cholesterol, diet, and coronary heart-disease:
an inter-racial survey in the Cape Peninsula. Lancet 1955; 269:
1103-8.
- Keys A, Anderson JT, Grande F. "Essential"
fatty acids, degree of unsaturation, and effect of corn (maize)
oil on the serum-cholesterol level in man. Lancet 1957a; 1: 66-8.
- Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol
responses of man to changes in fats in the diet. Lancet 1957b; 2:
959-66.
- Malmros H, Wigand G. The effect on serum-cholesterol
of diets containing different fats. Lancet 1957; 2: 1-7.
- Keys A, Anderson JT, Grande F. Serum cholesterol
in man: diet fat and intrinsic responsiveness. Circulation 1959;
19: 201-14.
- Keys A, Anderson JT, Grande F. Serum cholesterol
response to changes in the diet IV. Particular saturated fatty acids
in the diet. Metabolism 1965a; 14: 776-87.
- Hegsted DM, McGrandy RB, Myers ML, Stare FJ. Quantitative
effects of dietary fat on serum cholesterol in man. Am J Clin Nutr
1965; 17: 281-95.
- Hegsted DM. Serum-cholesterol response to dietary
cholesterol: a re-evaluation. Am J Clin Nutr 1986; 44: 299-305.
- Mensink RP, Katan MB. Effect of dietary fatty acids
on serum lipids and lipoproteins: A meta-analysis of 27 trials.
Arterioscl Thromb 1992; 12: 911-9.
- Keys A, Anderson JT, Grande F. Serum cholesterol
response to changes in the diet III. Differences among individuals.
Metabolism 1965b; 14: 766-75.
- Grundy SM, Vega GL. Plasma cholesterol resp 1000
onsiveness to saturated fatty acids. Am J Clin Nutr 1988; 47: 822-4.
- Katan MB, Berns MA, Glatz JF, Knuiman JT, Nobels
A, de Vries JH. Congruence of individual responsiveness to dietary
cholesterol and to saturated fat in humans. J Lipid Res 1988; 29:
883-92.
- Gustafsson I, Boberg J, Karlstrom B, Lithell M,
Vessby B. Similar serum lipoprotein reductions by lipid lowering
diets with different polyunsaturated: saturated fat values. Br J
Nutr 1983; 50: 531-7.
- Gustafsson I, Vessby B, Karlstrom B, Boberg J,
Boberg M, Lithell H. Effects on the serum lipoprotein concentrations
by lipid-lowering diets with different fatty acid compositions.
J Am College Nutr 1985; 4: 241-8.
- Gordon DJ, Salz KM, Roggenkamp KJ, Franklin FA
Jr. Dietary determinants of plasma cholesterol change in the recruitment
phase of the Lipid Research Clinics Coronary Primary Prevention
Trial. Arteriosclerosis 1982; 2: 537-48.
- McGandy RB, Hegsted DM, Myers ML. Use of semi-synthetic
fats in determining effects of specific dietary fatty acids on serum
lipids in man. Am J Clin Nutr 1970; 23: 1288-98.
- Tamamoto I, Sugano M, Wada M. Hypocholesterolaemic
effect of animal and plant fats in rats. Atherosclerosis 1971; 13:
171-84.
- Mensink RP, Zock PL, Katan MB, Hornstra G. Effect
of dietary cis and trans fatty acids on serum lipoprotein(a) levels
in humans. J Lipid Res 1992; 33: 1493-1501.
- Hayes KC, Pronczuk A, Lindsey S, Diersen-Schade
D. Dietary saturated fatty acids (12:0, 14:0, 16:0) differ in their
impact on plasma cholesterol and lipoproteins in non-human primates.
Am J Clin Nutr 1991; 53: 491-8.
- Hayes KC, Khosla P. Dietary fatty acid thresholds
and cholesterolaemia. FASEB J 1992; 6: 2600-7.
- Sundram K, Hayes KC, Siru OH. Dietary palmitic
acid results in lower serum cholesterol than does lauric-myristic
acid combination in normo lipidaemic humans. Am J Clin Nutr 1994;
59: 841-6.
- Bonanome A, Grundy SM. Effect of dietary stearic
acid on plasma cholesterol and lipoprotein levels. N Engl J Med
1988; 318: 1244-8.
- Beveridge JMR, Connell WF, Haust HL, Mayer GA.
Dietary cholesterol and plasma cholesterol levels in man. Can J
Biochem Physiol 1959; 37: 575-82.
- Hashim SA, Argeaga A, Van Itallie TB. Effect of
a saturated medium-chain triglyceride on serum-lipids in man . Lancet
1960; 1: 1105-8.
- Grundy SM. Saturated fat and coronary heart disease.
In: Winick M, ed. Nutrition and the killer diseases. New York: Wiley,
1981: 57-8.
- Grundy SM. Comparison of monounsaturated fatty
acids and carbohydrates for lowering plasma cholesterol. N Engl
J Med 1986; 314: 745-8.
- Grundy SM, Florentin L, Nix D, Whelan MF. Comparison
of monounsaturated fatty acids and carbohydrates for reducing raised
levels of plasma cholesterol in man. Am J Clin Nutr 1988; 47:965-9.
- Mensink RP, Katan MB. Effect of monounsaturated
fatty acids versus complex carbohydrates on high density lipoproteins
in healthy men and women. Lancet 1987; 1: 122-5.
- Harris WS, Connor WE, McMurray MP. The comparative
reductions of the plasma lipids and lip 1000 oproteins by dietary
polyunsaturated fats: salmon oil versus vegetable oils. Metabolism
1983; 32: 17984.
- Illingworth DR, Harris WS, Connor WE. Inhibition
of low density lipoprotein synthesis by dietary omega-3 fatty acids
in humans. Arteriosclerosis 1984; 4: 270-5.
- Nestel PJ. Nutritional control of cardiovascular
risk factors. Cardiovascular risk factors. Lipidology 1991; 1 (5):
259-64.
- Rogers AE, Connor B, Boulanger C, Lee S. Mammary
tumor-igenesis in rats fed diets high in lard. Lipids 1986; 21:
275-280.
- Connor WE. Hypolipidemic effects of dietary omega-3
fatty acids in normal and hyperlipidemic humans: effectiveness and
mechanisms. In: Simopoulos AP, Kifer RR, Martin RE, eds. Health
effects of polyunsaturated fatty acids in seafoods. New York: Academic
Press, 1986: 173-210.
- Vandongen R, Codde JP, Mori TA, Stanton KG, Masarei
JRL. Hypercholesterolaemic effect of fish oil in insulin-dependent
diabetics. Med J Aust 1988; 148: 141-3.
- Judd JT, Clevidence BA, Muesing RA, Wittes J, Sunkin
ME, Podczasy JJ. Dietary trans fatty acids: effects on plasma lipids
and lipoproteins of healthy men and women. Am J Clin Nutr 1994;
59: 861-8.
- Mensink RP, Katan MB. Effect of dietary trans fatty
acids on high-density and low-density lipoprotein cholesterol levels
in healthy subjects. N Engl J Med 1990; 323: 439-45.
- Nestel P, Noakes M, Belling B, et al. Plasma lipoprotein
lipid and Lp(a) changes with substitution of elaidic acid for oleic
acid in the diet. J Lipid Res 1992; 33: 1029-36.
- MacDonald I. Interrelationship between the influences
of dietary carbohydrates and fats on fasting serum lipids. Am J
Clin Nutr 1967; 20: 345-51.
- Descovich GC, Gaddi A, Mannino G, et al. Multicentre
study of soybean protein diet for outpatient hypercholesterolaemic
patients. Lancet 1980; 2: 709-12.
- Goldberg AP, Lim A, Kolar JB, Grundhauser JJ, Steinke
FH, Schonfeld G. Soybean protein independently lowers plasma cholesterol
levels in primary hypercholesterolemia. Atherosclerosis 1982; 43:
355-368.
- Sirtori CR, Gatti E, Mantero O, et al. Clinical
experience with the soybean protein diet in the treatment of hypercholesterolemia.
Am J Clin Nutr 1979; 32: 1645-58.
- Sirtori CR, Zucchi-Dentone C, Sirtori M, et al.
Cholesterol-lowering and HDL-raising properties of lecithinated
soy proteins in type II hyperlipidemic patients. Ann Nutr Metab
1985; 29: 348-57.
- Bodwell CE, Schuster EM, Steele PS, Judd JT, Smith
JC. Effects of dietary soy protein on plasma lipid profiles of adult
men. Fed Proc 1980; 39: 1113.
- Van Raaij JMA, Katan MB, Hautvast GAJ, Casein A,
Soya protein, serum-cholesterol. Lancet 1979; 2: 958.
- Wolfe BM, Taves EH, Giovannetti PM. Low protein
diet decreases serum cholesterol in healthy human subjects. Clin
Invest Med 1986; 9: A43.
- Lieber CS, Jones DP, Mendelson J, DeCarli LM. Fatty
liver, hyperlipemia, and hyperuricemia produced by prolonged alcohol
consumption, despite adequate dietary intake. Trans Assoc Am Physicians
1963; 76: 289-300.
- Glueck CJ, Heiss G, Morrison JA, Khoury P, 1000
Moore M. Alcohol intake, cigarette smoking and plasma lipids and
lipoproteins in 12-19-year-old children. The Collaborative Lipid
Research Clinics Prevalence Study. Circulation 1981; 64: 48-56.
- Barrett-Connor E, Suarez L. A community study of
alcohol and other factors associated with the distribution of high
density lipoprotein cholesterol in older vs. younger men. Am J Epidemiol
1982; 115: 888-93.
- Hartung GH, Forcyt JP, Mitchell RE, Mitchell JG,
Reeves RS, Gotto AM Jr. Effect of alcohol intake on high-density
lipoprotein cholesterol levels in runners and inactive men. J Am
Med Assoc 1983; 249: 747-50.
- Glueck CJ, Gordon DJ, Nelson JJ, Davis CE, Tyroler
HA. Dietary and other correlates of changes in total and low density
lipoprotein cholesterol in hypercholesterolemic men: the Lipid Research
Clinics Coronary Primary Prevention Trial. Am J Clin Nutr 1986;
44: 489-550.
- Anderson JW, Story L, Sieling B, Chen WJL, Petro
MS, Story J. Hypocholesterolemic effects of oat bran or bean intake
for hypercholesterolemic men. Am J Clin Nutr 1984; 40: 1146-55.
- Jenkins DJA, Reynolds D, Leeds AR, Waller AL, Cummings,
HH. Hypocholesterolemic action of dietary fiber unrelated to fecal
bulking effect. Am J Clin Nutr 1979; 32: 2430-5.
- Keys A, Anderson JT, Grande F. Diet-type (fats
constant) and blood lipids in man. J Nutr 1960; 70: 257-66.
- Jenkins DJA, Rainey-Macdonald CG, Jenkins AL, Benn
G. Fiber in the treatment of hyperlipidemia. In: Spiller GA, ed.
CRC handbook of dietary fiber in human nutrition. Boca Raton, Fl:
CRC Press 1986: 327-44.
- LSRO (Life Sciences Research Office). Physiological
Effects and Health Consequences of Dietary Fiber. Federation of
American Societies for Experimental Biology, Bethesda Md. 1987:
236.
- Anderson JW, Tetyen-Clark J. Dietary fiber: hyperlipidemia,
hyper-tension, and coronary heart disease. Am J Gastroenterol 1986;
81: 907-19.
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC,
Witzum JL. Beyond cholesterol: modifications of low-density lipoprotein
that increase its atherogenecity. N Eng J Med 1989; 320: 915-24
.
- Zock PL, Katan MB, Merkus MP, van Dusseldorp MV,
Heryvan JL. Effect of a lipid rich-fraction from boiled coffee on
serum cholesterol. Lancet 1990; 335: 1235-7.
- Kritchevsky D. The effect of dietary garlic on
the development of cardiovascular disease. Trends in Food Science
& Technology; June 1991; l41-4.
- Shao FC. Study of synthetic allicin on the prevention
and treatment of atherosclerosis. Acta Nutr Sinica 1982; 4: 109-16.
- Oakenfull D, Sidhu GS. Could saponins be a useful
treatment for hypercholesterolaemia? Eur J Clin Nut 1990; 44: 79-88.
- Qureshi AA, Qureshi N, Hasler-Rapacz JO, et al.
Dietary toco-trienols reduce concentrations of plasma cholesterol,
apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs
with inherited hyperlipidaemias. Am J Clin Nutr 1991; (Supp) 53:
1042S-6S.
- Qureshi AA, Qureshi N, Wright JJK, et al. Lowering
of serum cholesterol in hypercholesterolemic humans by tocotrienols
(Palmvitee). Amer J Clin Nutr 1991; (Supp) 53: 1021S-6S.
- Tilvis R S, Miettinen TA. Serum plant sterols and
their relation to cholesterol absorption. Am J Clin Nutr 1986; 43:
92-7.
- Wilcox G, Wahlqvist ML, Burger HG, Medley G. Oestrogenic
effects of plant foods in postmenopausal women. Br Med J 1990; 301:
905-6.
- Igarashi K, Inagaki K. Effects of the major anthocyanin
of wild grape (Vitis coignetiae) on serum lipid levels in rats.
Agric Biol Chem 1991; 55(1): 285-7.
- Igarashi K, Shinobu A, Satoh J. Effects of Atsumi-kabu
(Red Turnip, Brassica campestris L.) anthocyanin on serum cholesterol
levels in cholesterol-fed rats. Agric Biol Chem 1990; 54(1):171-175.
- Blankenhorn DH, Alaupovic P, Wickham E, Chin HP,
Azen SP. Prediction of angiographic change in native human coronary
arteries and aortocoronary bypass grafts: lipid and non-lipid factors.
Circulation 1990; 81 :470-6.
- Watts GF, Jackson P, Mandalia S, Lewis ES, Coltart
DJ, Lewis B. Nutrient intake and progression of coronary artery
disease. Am J Cardiol. 1994; 73: 320-32.
- Blankenhorn DH, Johnston RL, Mack WJ, Hafez A,
El Zein MD, Vailas LI. The influence of diet on the appearance of
new lesions in human coronary arteries. JAMA 1990; 263: 1646-52.
- Watts GF, Lewis B, Brunt JNH, et al. Effects on
coronary artery disease of lipid-lowering diet, or diet plus cholestyramine,
in the St Thomas’ Atherosclerosis Regression Study (STARS).
Lancet 1992; 339: 536-69.
- Arntzenius AC, Kromhout D, Barth JD, et al. Diet,
lipoproteins, and the progression of coronary atherosclerosis: the
Leiden Intervention Trial. N Engl J Med 1985; 312: 805- 11.
- Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle
changes reverse coronary heart disease? The Lifestyle Heart Trial.
Lancet 1990; 336: 129-33.
- Schuler G, Hambrecht R, Schlierf G, et al. Regular
physical exercise and low-fat diet. Effects on progression of coronary
artery disease. Circulation 1992; 86: 1- 11.
- Hodgson JM, Wahlqvist ML, Boxall JA, Balazs ND.
Can linoleic acid contribute to coronary artery disease? Am J Clin
Nutr 1993; 58: 228-34.
- Wood DA, Butler S, Riemersma RA, Thomson M, Oliver
MF. Adipose tissue and platelet fatty acids and coronary heart disease
in Scottish men. Lancet 1984; ii:117-21.
- Wood DA, Riemersma RA, Butler S, Thompson M, MacIntyre
C, Elton RA. Linoleic and eicosapentaenoic acids in adipose tissue
and platelets and risk of coronary heart disease. Lancet 1987; i:
177-83.
- Riemersma RA, Wood DA, Butler S, et al. Linoleic
acid in adipose tissue and coronary heart disease. Brit Med J 1986;
292: 1423-7.
- Dayton S, Pearce ML, Goldman H, et al. Controlled
trial of a diet high in unsaturated fat for prevention of atherosclerotic
complications. Lancet 1968; 2: 1060-2.
- Frantz ID Jr, Dawson EA, Ashman PL, et al. Test
of effect of lipid lowering by diet on cardiovascular risk. The
Minnesota Coronary Survey. Arteriosclerosis 1989; 9: 129-35.
- Miettinen M, Turpeinen O, Karvanon MJ, Elosuo R,
Paavilainen E. Effect of cholesterol-lowering diet on mortality
from coronary heart disease and other cau 1000 ses. A twelve-year
clinical trial in men and women. Lancet 1972; 2:835-8.
- Burr ML, Gilbert JF, Holliday RM, et al. Effects
of changes in fat, fish, and fibre intakes on death and myocardial
reinfarction: Diet and Reinfarction Trial (Dart). Lancet 1989; ii:757-61.
- de Logeril M, Renaud S, Manselle N, et al. Mediterranean
a -linolenic acid-rich diet in secondary prevention of coronary
artery disease. Lancet 1994; 343: 1454-9.
- Singh RB, Rastogi SS, Verma R, et al. Randomised
controlled trial of cardioprotective diet in patients with recent
acute myocardial infarction: results of one year follow up. Br Med
J.1992;304:1015-9.
- MRC, Research Committee to the Medical Research
Council. Controlled trial of soya-bean oil in myocardial infarction.
Lancet 1968; 2: 693-700.
- MRC, Research Committee to the Medical Research
Council. Low fat diet in myocardial infarction - a controlled trial.
Lancet 1965; 2: 501-4.
- Rose G, Thompson WB, Williams RT. Corn oil in treatment
of ischaemic heart disease. Br Med J 1965; 1: 1531-3
- Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist
C, Blacket RB. Low fat low cholesterol diet in secondary prevention
of coronary heart disease. Adv Exp Med Biol 1978; 109: 317-31
- Truswell AS. Review of Dietary Intervention Studies:
effect on coronary events and on total mortality. Australian NZ
J Med 1994; 24: 98-106.
- Kushi L, Lew RA, Stare FJ, et al. Diet and 20 yeas
mortality from coronary heart disease. The Ireland-Boston Diet-Heart
Study. New Engl J Med 1985; 312, 811-8.
- Kromhout D, Bosschieter EB, de Lezenne Coulander
C. Dietary fibre and 10-years mortality for coronary heart disease,
cancer and all causes. Lancet 1984;2:518-21.
- Lapidus L, Bengtsson C. Socioeconomic factors and
physical activity in relation to cardiovascular disease and health.
A 12-year follow-up of participants in a population study of women
in Gothenburg, Sweden. Br Heart J 1986; 55: 295-301.
- Morris JN, Marr JW, Clayton DG. Diet and heart:
a postscript. Br Med J 1977; 2: 1307-14.
- Kromhout D, Bosschieter EB, de Lezenne Coulander
C. The inverse relation between fish consumption and 20-year mortality
from coronary heart disease. N Engl J Med 1985; 312: 1205-9.
- Morrison LM. A nutritional program for prolongation
of life in coronary atherosclerosis. JAMA 1955; 159: 1425
- Leren P. The Oslo diet heart study: 11 year report.
Circulation 1970; 42: 935-42
- Bierenbaum ML, Fleischmann AI, Raichelson RI, Hayton
T, Watson PB. Ten year experience of modified fat diets on younger
men with coronary heart disease. Lancet 1973; i: 404-7.

Copyright © 1995 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
0
|