Asia Pacific J Clin Nutr (1995) 4: 259-264
Asia Pacific J Clin Nutr (1995) 4: 259-264

Diet and oral cancer - a case control
study
MPR. Prasad*, TP Krishna*, S Pasricha*,
MA Quereshi**, K Krishnaswamy*
*National Institute of Nutrition,
Hyderabad; **MNJ Cancer Hospital, Hyderabad
Apart from strong genotoxic carcinogens, other environmental
factors are implicated in both causes and prevention of cancers.
A hospital based case control study was conducted to examine the
role of diet in the aetiology of oral and oropharyngeal cancers.
In this article, past dietary intake and nutrient estimates, obtained
through diet history method and biochemical nutritional status at
the onset of the disease are presented. The results of the study
suggest that poor dietary intake of vegetables and fruits coupled
with low estimated intake of betacarotene, thiamine, riboflavin,
folate, vitamin C, iron and copper, modify the risk potential. The
biological indicators of the nutritional status such as plasma vitamin
A, E, red cell folate and plasma zinc were significantly reduced
in cases and yielded moderate risk estimates. The risk estimates
though of moderate magnitude are of importance in relatively homogeneous
subjects with respect to diet and nutrition.
The findings are in line with several other epidemiological
observations. The combined effects of micro nutrients appears to
be protective in countering the adverse effects of exogenous exposures
to tobacco. The protective role of vegetables and fruits is of potential
interest in terms of etiologic causes and prevention.
Introduction
Oral cancer is one of the ten most common cancers
in the world1. In India it accounts for a third of all
cancers2. It is documented in all parts of India. Tobacco
smoke and betel chewing with or without tobacco are suggested as etiologic
factors accounting for 90% of all oral cancersl. The mortality
and morbidity of oral cancers are well documented in India. In fact
tobacco related cancers seem to be a leading cause of cancers in the
whole of the Indian sub-continent2. The mortality of oral
cancers despite advances in treatment and management appear to be
high with poor chances of survival.
Though environmental exposure to tobacco and other
carcinogens are important determinants of cancer, it is now well documented
that other factors such as diet have an important role to play in
the multistage carcinogenesis process. Several dietary factors act
as risk modifiers3-7. In general though, dietary deficiencies
have not been shown to initiate events, epidemiological and experimental
studies provide strong evidence for dietary substance, in promotion,
progression and inhibition of cancer8,9.
World wide epidemiological data suggest a strong protective
role for fruits and vegetablesl0,ll. The protective effects
of these in addition to non nutrients have been attributed to micronutrients
s 1000 uch as vitamin A, b carotene, folate, vitamin E and
vitamin C12. Limited observation suggest that probably
zinc and selenium deficiencies are also causally relatedl3-14.
The surveys conducted by NNMB in 10 states of India
document poor consumption of green leafy vegetables and also poor
intake of vitamins A, C, folate and riboflavin in population groups15.
As early as 1933, Orr in his classical description of oral cancer
in India attributed vitamin A deficiency as one of the important contributing
agents16. As there is meagre information on the dietary
and nutritional correlates of oral cancer in India, a case control
study of oral and oropharyngeal cancers was envisaged. The objectives
of the study were to :
- quantitate past dietary intake of food groups and
nutrients
- estimate the nutritional status at the onset of
the disease and arrive at risk estimates.
Materials and methods
Cases :
The cases were patients of oral cancer, who presented
themselves for the first time at the Government Cancer Hospital (Mehdi
Nawab Jung) Hyderabad. Advanced cases and those who received treatment
elsewhere were deleted. In all, 45 subjects of both sexes were included
after excluding those who had previously consumed vitamin or mineral
pills for several days.
Controls:
The controls were carefully matched for age, sex,
socioeconomic status and habits and were drawn from attendants of
the cancer patients or from patients suffering from non cancerous
or other minor ailments.
A detailed clinical, anthropometric and personal history
was taken and 10ml blood sample collected in an heparanised tube for
biochemical analysis. A dietary history, using standard dietary cups
were obtained from compliant individuals participating in the study.
Dietary details:
Dietary consumption pattern over the last six months
was assessed by an oral questionnaire. The questionnaire was designed
to obtain information regarding the dietary habits. Average frequency
of consumption of food items in the preceding six months or before
the change in the dietary habit and quantities of different food groups
were obtained. From the frequency of consumption, number of food items
consumed per month were obtained.
Biochemical Analysis:
Haemoglobin and albumin were estimated by cyanmethaemoglobin
and dye binding methods respectively17,18. RBC folate was
estimated micro-biologically using L. Casei19. Riboflavin
and thiamin levels were estimated indirectly by measuring the erythrocyte
glutathione reductase (EGR) and erythrocyte transketolase (ETK) activity
and expressed as activity coefficients20,21. High pressure
liquid chromatography was used for plasma vitamin A, vitamin E was
determined by thin layer chromatography22,23. Serum iron
was estimated colorimetrically while the trace metals, copper, magnesium
and zinc were estimated by atomic absorption spectrophotometry24,25.
Statistical Analysis:
The Mann-Whitney test was applied for comparisons
of means between the two groups26. Odds ratios (ORs) were
computed (using control values as cut off points) after the intake
of nutrients were adjusted for total calorie intake27,28.
Wherever significant odds ratios were observed, 95% confidence intervals
(CI) were also included.
Results
The 1000 mean age in our study was 54 years. More
than 90% were either tobacco smokers or tobacco chewers. Smoking was
a consistent feature. None of the patients had the habit of reverse
smoking. The distribution of cancers by sites are given in Table 1.
The body weights of patients varied between 31.0 to 67.0kgs and a
mean weight of 45.2± 1.29kgs and the mean body mass index (BMI)
was 0.17± 0.003. The controls had a mean weight of 49.3± 1.38kgs and mean BMI of 0.19± 0.005 as shown in Table 2.
Table 1. Age, weight, site
of cancer, and TNM classification
| |
Cases
|
Controls
|
| Age (years) |
53.9± 1.7
|
53.5± 1.8
|
| Weight (kg) |
45.2± 1.29
|
49.3± 1.38
|
| Site of cancer |
| a) Tongue |
18
|
|
| b) Oral cavity |
19
|
|
| c) Oropharynx |
8
|
|
|
Number of cases by TNM classification
|
| T1N0M0 |
8
|
|
T1N1M0
T2N0M0 |
10
|
|
T2N1M0
T2N2M0 |
20
|
|
T2N1M1
T2N2M1 |
2
|
|
T3N1M0
T3N3M0 |
5
|
|
Values Mean ± SD. No. of subjects in each group
= 45
|
Table 2. Blood nutrients and
other parameters
| Parameter |
Case
|
Control
|
| Body mass index |
0.17± 0.003**
|
0.19± 0.005
|
| Haemoglobin g/
l |
120.5± 1.97
|
123.2± 1.85
|
| Albumin g/ l |
32.3± 0. 1000 45**
|
34.6± 0.40
|
| RBC folate nmol/
l |
277.4± 4.79*
|
295.3± 6.54
|
| EGR (AC) |
1.27± 0.019
|
1.25± 0.018
|
| ETK (AC) |
1.22± 0.019
|
1.20± 0.02
|
| Vit A m g/ dl |
27.3± 1.9**
|
39.6± 1.66
|
| Vit E m g/ dl |
570.0± 34.5***
|
920.6± 57.97
|
| Serum iron m mol/ l |
16.5± 0.94
|
16.9± 0.47
|
| Copper m mol/ l |
19.9± 0.35
|
19.9± 0.50
|
| Magnesium m mol/ l |
1.4± 0.037**
|
1.2± 0.033
|
| Zinc m mol/ l |
9.8± 0.21***
|
14.2± 0.58
|
Values are mean ± SE. No. of observations 45 in each
group
* p<0.02 **p<0.005 ***p<0.001
|
The protein energy status as reflected by serum albumin
was significantly lower than that of controls (Table 2). More than
60% of the cases were in first tertile (Fig. 1). The haemoglobin levels
and the serum iron status were not different between cases and controls.
Figure 1. Tertile distribution of percent cases
and controls for serum albumin. (p<0.05) and RBC folates (p<
0.05)
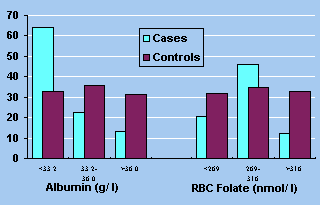
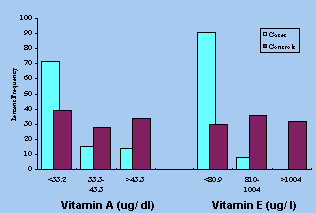
Figure 2. Tertile distribution of percent cases
and controls for plasma vitamin A (p<0.01) and vitamin E (p<
0.001).
However, of the B-complex vitamins, red cell folate
was significantly lower in cases (p <0.02). The status of other
B complex vitamins, viz. thiamin and riboflavin were not affected.
Among fat soluble vitamins both vitamin A (p <0.005)
and vitamin E (p <0.001) were significantly low. The tertile distribution
indicated in Figure 2 reflects the same.
The two trace metals magnesium and zinc were significantly
different from the controls with the former being higher while the
latter was lower in cases, with all the case subjects falling in the
first tertile (Figure 3). Serum copper level was not affected.
Figure 3. Tertile distribution of percent cases
and controls for plasma magnesium (p <0.02) and zinc (p <0.001).
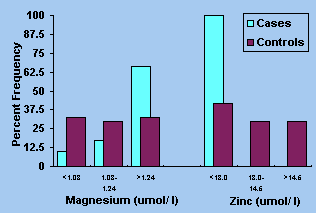
| The odds ratios in Table
3 indicated that the risk was higher for low haemo 1000 globin,
albumin, folate and vitamin A; whereas the risk was higher for
high magnesium levels. Plasma zinc and vitamin E values were not
presented in the table as the populations of the two groups were
well apart giving an infinite odds ratio.
Dietary assessment
Food Groups
The major food groups such as cereals, pulses,
milk, vegetables and fruits were quantitated (Table 4). The
vegetable intake green or otherwise was significantly low as
compared to controls. In fact, the intake of both these food
groups was only a third to half of that observed in controls.
When the frequencies of several food items (Table
5) were analysed, more frequent intake of other vegetables were
associated with lower risk of oral cancer (p <0.01), while
the OR indicated a higher risk with lower consumption of vegetables
and higher consumption of flesh and fried foods.
Nutrients
The nutrient intakes were computed as per the
Nutritive Value of Indian Foods from the diet history. It was
obvious that the cases and the controls were taking many fewer
calories (Table 6)29. When all the nutrients were
adjusted to the energy intake, the intake of carotene, riboflavin
and magnesium were significantly higher in controls. The odds
ratios show higher risk associated with low intakes of carotene,
thiamin, riboflavin, folic acid, vitamin C, iron, magnesium
and copper.
Discussion
Epidemiological observations throughout the
world clearly demonstrate that environmental exposure to tobacco
is an important factor in the aetiology of oral cancerl.
Literary evidences document not only an association between
tobacco and oral cancers but also highlight the risk modifying
properties of certain specific food items such as vegetables
and fruits and also of nutrients such as vitamin A, C and betacarotene30-32.
Macro and micro nutrient deficiencies or imbalances have long
been considered as probable causes or risk modifiers of cancer
of the oral cavity33. Our study aimed to investigate
the influence of dietary habits on the risk of tobacco induced
oral and oropharyngeal cancers which were diagnosed within 2
months of the onset of signs and symptoms. It provided an opportunity
to study the role of diet in the aetiology of oral and oropharyngeal
cancers. The tumour node metastasis (TNM) classification, suggest
that most of them were diagnosed in the early stages of tumour
development with or without the involvement of local lymph nodes
with a few exceptions.
The findings of the present study either on
the dietary intake prior to the onset of the disease or the
nutrient profile at the onset, may be considered as indicators
of natural sequence in the evolution of disease process, as
not all nutrients are affected but only those that are aetiologically
related.
The general nutritional status of the cases
as reflected by body weight, BMI and albumin, appeared to be
lower than the controls. We have earlier documented that protein
energy malnourished individuals were at greater risk for developing
cancers as they have a higher metabolic susceptibility to carcinogen
such as polycyclic aromatic hydrocarbons34-37.
Th 1000 e nutrient profiles estimated at the
onset of the disease, indicated that vitamin A, E, zinc and
folate-deficient subjects were susceptible to cancers. Several
investigators have reported the protective effects offered by
vitamins A and C rich foods38-40. The fact that in
the present study the estimates of prior dietary intake indicated
low intakes of betacarotene, riboflavin and vitamin C rich foods,
favours the causal relationship of the nutrient deficiencies
to the development of the disease process. The relative risk
estimates, though of moderate magnitude, further reinforce the
aetiological relationships as the subjects investigated were
relatively homogeneous with respect to dietary intake.
|
Table 3. Odds ratios (ORs)
with 95% confidence intervals (CI) for oral cancer by tertile
by nutrients in blood
| Nutrient |
OR
|
CI
|
| Haemoglobin |
1.00
2.57
2.86
|
(0.82-8.08)
(0.92-8.90)
|
| Albumin |
1.00
1.44
4.33
|
(0.41-5.05)
(1.36-13.82)
|
| RBC Folates |
1.00
3.92
3.80
|
(1.16-13.240)
(1.11-13.030)
|
| EGR |
1.00
0.76
1.45
|
(0.27-2.15)
(0.53-3.96)
|
| ETK |
1.00
1.90
1.32
|
(0.67-5.40)
(0.48-3.59)
|
| Vitamin A |
1.00
1.38
4.62
|
(0.36-5.35)
(1.47-14.52)
|
| Iron |
1.00
0.27
0.87
|
(0.08-0.95)
(0.31-2.42)
|
| Copper |
1.00
0.42
0.87
|
(0.14-1.23)
(0.31-2.43)
|
| Magnesium* |
1.00
1.73
6.00
|
(0.47-6.38)
(1.77-20.31)
|
*The comparison in this is done with 1st tertile
value.
No. of observations = 45
Table 4. Average intake (g) of food groups
| Foods
groups |
Cases (16)
|
Controls (16)
|
| Cereals |
259± 33.0
|
313± 32.7
|
| Pulses |
23.4± 4.34
|
24.8± 4.56
|
| Green leafy vegetables |
9.09± 0.97
|
26.29± 8.08*
|
| Other vegetables |
50.1± 11.79
|
106.4± 19.45**
|
| Milk & milk
products |
84.3± 16.23
|
70.8± 18.06
|
| Flesh foods |
15.4± 6.08
|
10.3± 3.18
|
| Poultry products |
8.1± 6.12
|
7.5± 2.39
|
| Fruits |
24.4± 18.09
|
53.9± 13.56
|
| Oils/ fats |
15.6± 2.66
|
13.9± 1.89
|
Figures in parenthesis indicate no. of observations.
All values are mean ± SE *P <0.05 **P <0.02
|
Table 5. Mean frequency of intake
of food groups/ month with odds ratios (ORs) and 95% confidence intervals
(CI) for oral c 1000 ancer
| Foods |
Cases*
|
Controls*
|
ORa
|
CI
|
| Cereals |
75.2± 7.8
|
86.3± 3.8
|
0.43
|
|
| Pulses |
26.8± 5.3
|
31.9± 6.6
|
1.00
|
|
| Green leafy
vegetables |
5.93± 2.0
|
6.9± 2.0
|
1.80
|
0.39-8.21
|
| Other vegetables,
roots & tubers |
20.7± 3.6
|
46.6± 6.2**
|
¥
|
|
| Milk and
milk products |
9.4± 3.5
|
6.9± 3.8
|
0.51
|
|
| Animal
foods |
4.9± 1.9
|
4.2± 0.99
|
2.60
|
0.52-13.04
|
| Fruits |
7.0± 2.2
|
7.5± 2.2
|
1.29
|
|
| Fried foods |
16.2± 5.2
|
3.3± 2.0
|
--
|
|
| Fish |
0.75± 0.28
|
0.19± 0.13
|
0.18
|
|
| Tea/ coffee |
61.9± 9.6
|
37.5± 7.5
|
0.20
|
|
| Fats and
oils |
1000
0.6± 0.24
|
0.5± 0.31
|
0.21
|
|
| Spices |
34.5± 5.8
|
31.5± 6.3
|
0.89
|
|
No. of subjects in each group = 16. a:
Median of the control taken as the cut-off point. *Mean± SE ** p< 0.01
Table 6. Calorie adjusted mean
nutrient intake and odds ratio (ORs) with 95% confidence intervals
(CI) for oral cancer
| Nutrients |
Casesb
|
Controlsb
|
ORa
|
CI
|
| Calories |
1938.21± 0.34
|
1984.27± 0.34
|
--
|
--
|
| Proteins
(g) |
49.85± 0.30
|
47.20± 0.28
|
0.45
|
--
|
| Fat (g) |
35.55± 0.42
|
28.56± 0.42
|
0.45
|
--
|
| Calcium
(mg) |
545.00± 0.57
|
596.90± 0.45
|
1.29
|
|
| Carotenea
(mg) |
330.67± 0.50
|
655.59± 0.56*
|
3.00
|
0.67-13.40
|
| Thiamin
(mg) |
0.68± 0.43
|
0.81± 0.35
|
2.20
|
0.52-9.30
|
| Riboflavin
(mg) |
0.66± 0.38
|
1.74± 1.48*
|
4.33
|
0.88-21.30
|
| Folic acid
(m g) |
103.75± 0.40
|
128.65± 0.36
|
4.33
|
0.88-21.30
|
| Vitamin
C (mg) |
39.66± 0.52
|
43.68± 0.59
|
2.20
|
0.52-9.30
|
| Iron (mg) |
21.26± 0.30
|
22.49± 0.30
|
1.67
|
0.41-6.82
|
| Magnesium
(mg) |
217.22± 0.40
|
327.49± 0.33**
|
5.57
|
1.13-27.52
|
| Copper
(mg) |
3.05± 0.43
|
3.91± 0.32
|
4.33
|
0.88-21.31
|
| Zinc (m g) |
5308.8± 0.51
|
5766.3± 0.30
|
0.78
|
|
No. of observations in each group = 16;
a: negligible amount of vitamin A present (not included in carotene);
b:Mean± SE; *p< 0.05; **p< 0.01
It is interesting to note that dietary sources for
betacarotene and folate are similar, namely vegetables, of which intake
appears to be low. There is evidence to show that a precancerous condition
has been associated with low folate intake41. Population
based registries in India document a higher incidence of cervical
cancers42. It is possible that folate in general may be
involved in differentiation of epithelial cells as cancers at the
above sites were all squamous cell carcinomas. Our observation on
oesophageal cancer indicated low red cell folate levels. Infants in
India are born with inadequate stores of folate and several other
nutrients and continue to subsist on inadequate intakes of micro nutrients42,43.
The sources of vitamin E in the Indian diet are vegetable
oils and cereals. Though the intakes of these two items were not significantly
different, the intakes in general, in both cases and controls were
much less than the recommended dietary allowances. It is possible
that in cases where other nutrient deficiencies exist, the prooxidant
and antioxidant balance is tilted towards adverse effect. Current
research evidences support a strong preventive role for all antioxidants
mentioned above in the causation of cancers at different sites. It
was therefore not surprising to observe that vitamins A, C, E and
betacarotene appeared to be protective in our series of cases. In
the multistage carcinogenic process, which is complex, antioxidants
appear to function as an inhibitor of promotional aspects of cancer44.
In our earlier study on oesophageal cancers, results
indicated a greater risk in individuals with low plasma zinc in addition
to low vitamin A levels45. The discovery of the role of
zinc in cancer is recent. Plasma zinc is known to decrease in several
cancers13. Studies in China, Africa, Iran and Russia show
a positive association between dietary zinc deficiency and higher
incidence of oesophageal carcinomas46-51. Our study is
one of the first to highlight an association between plasma zinc and
oral cancer. The Indian diets which are cereal and pulse based are
higher in phytates which can decrease absorption of zinc and contribute
towards a zinc deficient state52. The intake of zinc particularly
in the low income group is half of the recommended dietary allowance
for Indians29.
The experimental findings in relation to chemical
carcinogenesis support our present observations of higher risk for
zinc-deficient subjects particularly for nitrosamine induced cancers53.
Zinc is known to have a significant effect on glutathione-S transferase
and also on DNA repair mechanisms54.
The relation of oral cancer with magnesium is intriguing.
We have no satisfactory explanation for higher plasma magnesium levels
in cases as compared to controls. The other non-nutrient dietary items
examined were in line with the observations of Notani and Jayant on
upper aerodigestive cancers wherein more frequent co 1000 nsumption
of tea/ coffee, chillies and less frequent intake of fish appeared
to increase the risk55.
We are aware of the limitation in interpretation of
our results. Familiarity with the hypothesis is a potent source of
bias. Though the relationship between dietary constituents (nutrients
and non nutrients) and cancer is complex, the consistent observation
across wide population groups in different countries strongly support
vitamins A, C and betacarotene as candidates for chemoprevention agents56,57.
Our findings must be assessed in this context as the results are in
agreement with previous epidemiological work in several countries7,30,38.
Thus the micronutrients in the diet appear to play
a pivotal role in reducing damage due to environmental exposures and
probably act synergistically to enhance several protective mechanisms
against carcinogenesis.
Acknowledgements
We thank Dr. Vinodini Reddy, Director, National Institute
of Nutrition for her keen interest and valuable suggestions. We thank
Mr KP Dalvi, Mr VK Goud, Mr PN Rao for their excellent technical assistance.
Diet and oral cancer - a case control
study
MPR. Prasad, TP Krishna, S Pasricha,
MA Quereshi, K Krishnaswamy
Asia Pacific Journal of Clinical
Nutrition (1995) Volume 4, Number 2: 259-264

References
- World Health Organization, Control of oral cancer
in developing countries-- a WHO Meeting. Bulletin of WHO Geneva
1984; 62: 817-830.
- Annual Report, Population cancer registries-- New
Delhi: Indian Council of Medical Research, 1989.
- Micozzi MS. Foods, micronutrients, and reduction
of human cancer. In: Moon TE, Micozzi MS, eds. Nutrition and cancer
prevention. New York Marcel Dekker Inc, 1989: 213-242.
- Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids
as preventive and therapeutic anticancer agents (Part I). Cancer
Treat Rep 1987; 71: 391-405.
- Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids
as preventive and therapeutic anticancer agents (Part II). Cancer
Treat Rep 1987; 71: 493-515.
- Tuyns AJ, Riboli E, Doornbos G. Nutrition and cancer
of the oesophagus. In: Jossens JV, Hill MJ, Geboers J, eds. Diet
and human carcinogenesis, Amsterdam Elsevier, 1985; 71-79.
- Marshall J, Graham S, Mettlin C, Shedd D, Swanson
M. Diet in the epidemiology of oral cancer. Nutr Cancer 1982; 3:
145-149.
- Newberne PM, Schrager TF, Canner MW. Experimental
evidence on the nutritional prevention of cancer. In: Moon TE, Micozzi
MS, eds. Nutrition and cancer prevention. New York Marcel Dekker
Inc, 1989: 33-82.
- Hunter DJ, Willet WC. Human epidemiological evidence
on the nutritional prevention of cancer. In: Moon TE, Micozzi MS,
eds. Nutrition and cancer prevention. New York Marcel Dekker Inc,
1989: 83-100.
- Steinmetz KA, Potter JD. Vegetables, fruit and
cancer. I, Epidemiology. Cancer causes and control, 1991; 2: 325-327.
- Ziegler RG. Vegetables, fruits and carotenoids
and the risk of cancer. Am J Clin Nutr 1991; 53: 251s-259