Asia Pacific J Clin Nutr (1995) 4: 251-255
Asia Pacific J Clin Nutr (1995) 4: 251-255

Physiological differences of soluble
and insoluble dietary fibre fractions of brown algae and mushrooms
in pepsin activity in vitro and protein digestibility
Y. Horie*, K. Sugase** and K. Horie**
* Nagoya Municipal Women's Junior
College, Nagoya, Japan; **Aichi Gakusen Women's Junior College, Okazaki,
Aichi, Japan.
This study was
presented in part at the 15th International Congress of Nutrition
on, Adelaide, Australia, Sept, 1993.
Soluble and insoluble dietary fibre fractions were
separated from Konbu, Wakame and Hijiki seaweeds and Shiitake, Hiratake
and Yanagimatsutake mushrooms, respectively, and the effects of the
fractions on pepsin activity in vitro and of those from Wakame on
apparent protein digestibility in vivo were studied. Addition of each
dietary fibre fraction inhibited pepsin activity in vitro in all the
dietary fibre fractions tested, particularly the inhibition by soluble
dietary fibre fractions being significantly greater, by 62-99%, than
that by insoluble dietary fibre fractions, by 22-36% (P< 0.01 in
each food). This suggests that soluble dietary fibres in algae and
mushrooms are likely to play a different physiological role from insoluble
dietary fibres. Measurement of viscosity of each soluble dietary fibre
fraction resulted in the correlation of viscosity with the inhibition
of pepsin activity by the soluble fraction. Young adult rats given
a normal protein diet containing 5% of the soluble dietary fibre fraction
derived from the Wakame seaweed showed a greater decrease in apparent
protein digestibility by 9.4% than those given the diet containing
5% of the insoluble one (P< 0.01). This may have resulted in the
significantly lower body weight gain of the former rats than that
of the latter rats.
Introduction
We recently reported1 that the soluble,
insoluble and total dietary fibre contents of some traditional Japanese
seaweeds and mushrooms were determined using the method of Prosky
et al.2 without the enzymes involved. The seaweeds showed
high values of soluble dietary fibre, 40-60% on dry matter basis,
while the mushrooms showed high values of insoluble dietary fibre,
more than 90%. Some purified soluble dietary fibres are likely to
exert physiological effects which are different from those of insoluble
dietary fibres3-6, and are summarised to have inhibitory
effects on digestive proteolytic enzymes and protein digestibility
in vitro7,8. Further, it has been suggested that the purified
soluble dietary fibres with viscous property might have the inhibitory
potency of protein digestion6,9. However, the physiological
effects of mixed soluble or insoluble dietary fibres which could be
separated from seaweeds and mushrooms are not well documented.
In the present study, soluble and insoluble dietary
fibre fractions were separated from three seaweeds-- Konbu, Wakame
and Hijiki, and from three mushrooms-- Shiitake, Hiratake and Yanagimatsutake,
based on 1000 the method of Prosky et al.2 modified by
the authors1. The effect of each dietary fibre fraction
on pepsin activity in vitro was examined. Then, viscosity of each
soluble fraction was measured in relation to the inhibition of the
pepsin activity in vitro. Further, to test the in vivo effect of the
dietary fibre fractions, apparent protein digestibility was measured
in rats fed normal protein diets containing the soluble or insoluble
dietary fibre fraction separated from the Wakame seaweed.
Methods
Separation and purity of soluble and insoluble
dietary fibre fractions
Separation of both fractions was carried out using
the method of Prosky et al.2 modified for algae and mushrooms
by the authors1 with no use of enzymes needed, which is
useful to control contamination of the enzymes used in the fractions.
Three traditional Japanese foods of brown algae, Konbu, Wakame and
Hijiki, packed in the dried state, and three of raw mushrooms, Shiitake,
Hiratake and Yanagimatsutake, were purchased at shops in Nagoya and
Okazaki in Japan. The brown algae, as they were, and the fungi, after
freeze-drying, were milled to pass through a 0.35mm mesh sieve and
kept in a dessicator. The powdered sample with 0.05M phosphate buffer,
pH 6.0,(brown algae, 0.3%; mushrooms, 2%) were heated at 97°C for
30 minutes. After being adjusted to pH 4.5 with phosphoric acid, the
contents were centrifuged at 3,000 rpm, the supernatants, precipitated
with 95% ethanol (60°C), were centrifuged, washed with ethanol and
acetone and dried for soluble dietary fibre fraction (SDF). Residues
were washed with ethanol and acetone and dried for insoluble dietary
fibre fraction (IDF).
Each dried fraction contained considerable amounts
of Kjeldahl nitrogen, 0.5-1.0% for SDFs and 2.4-6.1% for IDFs, from
the brown algae, and 2.4-4.0% for SDFs and 2.7-6.7% for IDFs, from
the mushrooms. Treatment of each fraction with protease (No.A-3910,
Sigma) for four hours showed no reduction of its Kjeldahl nitrogen
content, which probably indicates that the nitrogen in the SDFs and
IDFs is not derived from protein but presumably from nitrogen containing
dietary fibre such as chitin. Considerable amounts of chitin, which
is a nitrogen containing dietary fibre, in the mushrooms, 8.88% for
Shiitake, 4.99% for Hiratake, are reported1. The nitrogen
content of ethanol precipitates from Shiitake fungus1,
0.72% is nearly consistent with the chitin nitrogen. 0.61%10,
on dry weight basis of the food.
Pepsin activity in vitro determination
Pepsin activity was measured basically by the method
of Anson11 with minor modifications12. The enzyme
system of 2ml of pepsin aqueous solution with 1ml of 2% haemoglobin
in 0.06N HCl (pH 1.5) was incubated at 37°C for 3 minutes. After stopping
the reaction with the addition of 5% trichloroacetic acid, the contents
were centrifuged and the phenol reagent positive materials of the
supernatant were determined colorimetrically to serve as controls.
To test the effect of each dietary fibre fraction, powdered dietary
fibre fraction, 5 to 15mg from brown algae, 5 to 30mg from mushrooms
were added to the enzyme solution. The SDF from the seaweeds made
the solution nearly solid at more than 10mg added, and the SDF from
the mushrooms did so at more than 15mg. The pepsin activity was assayed
in the same manner as described above. Each assay was carried out
in quadruplicate. We expressed here a decrease in pepsin activity
as an ‘inhibition’ since Houck7 had evidenced
enzymologically that carrageenan were able to inhibit the pepsin activity
in vitro. An inhibition rate (%) was computed by subtracting the latter
activity from the controls.
Measurement of vi 1000 scosity of the soluble dietary
fibre fractions
Viscosity of samples was measured by the E Type Viscometer
(Toki Sangyo, Tokyo, Japan) in the conditions close to the pepsin
solution. Each sample was made a solution or a suspension in 1ml of
water at 37°C and its viscosity was measured. The rotatory speed of
the corn, 50rpm was adequate. Samples of insoluble dietary fibre fraction
were impossible to measure. The value for viscosity was expressed
as m Pa.s (mega Pascal second).
Measurement of apparent protein digestibility in
rats Fourteen young adult rats of Wistar strain were purchased
commercially. They were housed in individual, suspended, wire-mesh
stainless cages in a room maintained at approximately 25°C with alternate
12-hour periods of light and dark. The rats were provided diets and
water ad libitum. They were fed a basal dietl3 containing
20% of casein until they attained a body weight of 270 to 300g, then
divided randomly into two groups of 7 animals each. Rats of each group
were given the normal protein diet containing 5% of SDF or IDF fraction
separated from Wakame seaweed for seven days. The test diets contained
the following ingredients, as percentage weights: fe-starch from potato,
60; casein, 20; corn oil containing 100mg of vitamin E, 10; mineral
mixturel3, 4; vitamin mixturel3, 0.85; choline chloride 0.15; SDF
or IDF fraction, 5. Food intakes for the last three days were recorded,
and faeces excreted for the same period were collected in metabolic
cages. Their nitrogen contents were analysed by the semi-micro Kjeldahl
method. To determine apparent digestibility of dietary protein, absorbed
nitrogen and intake of protein nitrogen were computed using the measured
nitrogen of faeces excreted and that of food intake. The true nitrogen
intake for dietary protein was estimated by subtracting the nitrogen
from SDF or IDF contained in the diet from the total nitrogen intake.
Results and discussion
Effect of dietary fibre fractions on pepsin activity
in vitro
When 5 to 15mg of the SDFs or IDFs from the three
seaweeds were added to the enzyme system, pepsin activity in vitro
was inhibited as the added amount increased (Figure 1). The inhibition
was significantly greater in the SDFs than in the IDFs of the seaweeds
(P< 0.01), and particularly, the inhibition by the Konbu’s
SDF was greatest amongst the seaweeds tested. A similar relation was
seen in the three mushrooms when 5 to 30mg of each dietary fibre fraction
was added to the enzyme system (Figure 2). The inhibition by the Hiratake’s
SDF was greatest amongst the mushrooms tested. The strong inhibition
of pepsin by the soluble fractions is similar to another finding7
regarding a purified soluble dietary fibre of carrageenin inhibiting
pepsin activity in vitro.
Figure 1. Inhibitory effects of adding soluble
(SDF and insoluble (IDF) dietary fibre (DF) fractions separated from
Konbu, Wakame and Hijiki on pepsin activity.

Five to 15mg of each powdered fraction was added to the enzyme system,
which was incubated for 3 minutes at 37o C at pH 1.5. The
inhibition by SDF was significantly higher (p < 0.01, by t-test)
than by IDF in each food. Vertical lines indicate standard deviation.
Figure 2. Inhibitory effects of adding soluble
(SDF) and insoluble(IDF) dietary fibre (DF) fractions separated from
Shiitake, Hiratake and Yanagimatsutake on pepsin activity.
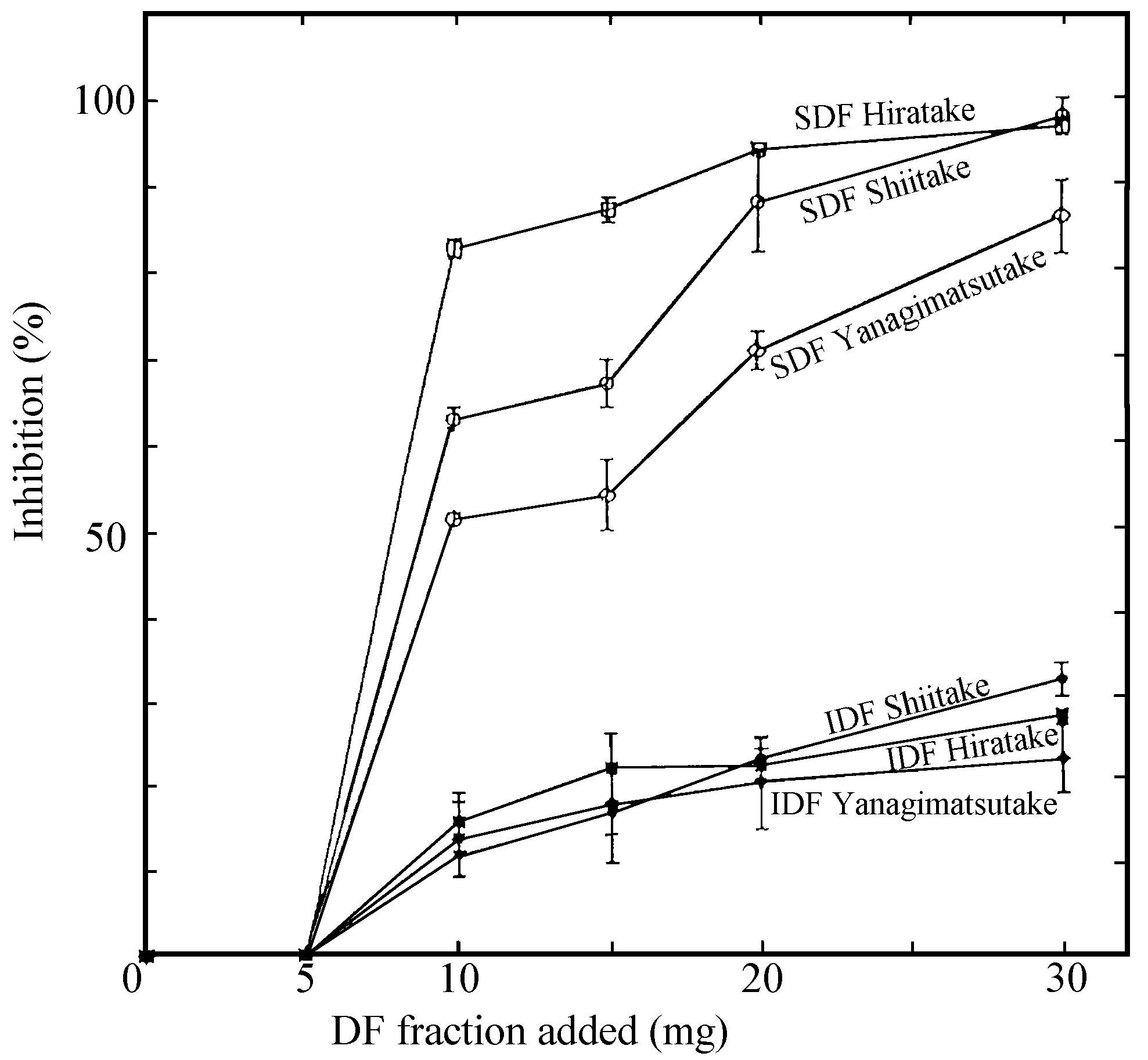
Ten to 30 mg of each powdered fraction was added to
t 1000 he enzyme system which was incubated for 3 minutes at 37o
C at pH 1.5. The inhibition by SDF was significantly higher
(p< 0.01, by t-test) than by IDF in each food. Vertical lines indicate
standard deviation.
The authors observed that the addition of dried powders
of the seaweeds and mushrooms (final concentration of each powder
in the enzyme system, 5% w/ v) inhibited the enzyme activityl2.
The inhibitions by the three seaweed powders were significantly greater
than those by the three mushroom powders. This can be elucidated by
the effects of the SDFs and IDFs involved in these foods. Thus, the
soluble dietary fibres of brown algae and mushrooms are considered
to have different inhibitory roles from insoluble dietary fibres in
protein availability in the gastrointestinal tracts. However, whether
the inhibition of pepsin activity in vitro is related or not to a
decrease in protein digestibility in vivo remains unclear.
Correlation of the pepsin activity inhibitions
by soluble dietary fibre fractions to their viscosities
As shown in Table 1, the higher the concentration
of SDF in the enzyme system, the greater its viscosity and pepsin
activity inhibition. Consequently, a close correlation has been observed
between viscosity and pepsin activity inhibition in the six foods.
The inhibition of pepsin activity in vitro by soluble dietary fibre
fractions might be attributed to their viscous property. We demonstrated
here the association between SDF and IDF preparations or the viscosity
of SDF preparations and the in vitro inhibition of pepsin. However,
there is a possibility that both prep-arations might be contaminated
with cell wall components which might be responsible for the observed
inhibition of the enzyme or viscosity. However, we have no suitable
method to detect and separate them from dietary fibres at present.
This deserves further investigation.
Table 1. Viscosities and inhibitions
of pepsin activity in vitro by the soluble dietary fibre fractions
from seaweeds and mushrooms as a function of concentration of sample
added and their relations.
| Food Name |
|
Concentration of sample added
|
Correlation!
|
| Seaweeds: |
|
0.15%
|
0.25%
|
0.35%
|
0.50%
|
0.75%
|
|
| Konbu |
Viscosity (x)*
|
1.632
|
1000
2.076
|
2.928
|
4.308
|
7.332
|
y = 52.2 + 5.25x
|
| |
Inhibition (y)#
|
|
55.3
|
|
98.9
|
99.1
|
g = 1.862 !
|
| Wakame |
Viscosity (x)
|
|
3.072
|
6.072
|
11.39
|
|
y = 16.6 + 2.19
|
| |
Inhibition (y)
|
|
21.0
|
|
45.8
|
61.6
|
g = 0.983
|
| Hijiki |
Viscosity (x)
|
|
0.888
|
0.996
|
1.152
|
1.296
|
y = -52.4 + 10 1000 2.4x
|
| |
Inhibition (y)
|
|
41.3
|
|
57.8
|
85.4
|
g = 0.952
|
| Mushrooms: |
|
0.25%
|
0.50%
|
0.75%
|
1.00%
|
1.50%
|
|
| Shiitake |
Viscosity (x)
|
|
3.012
|
3.672
|
5.280
|
8.220
|
y = 28.5 + 22.9x
|
| |
Inhibition (y)
|
0.00
|
63.0
|
67.1
|
88.0
|
97.8
|
g = 0.954
|
| Hiratake |
Viscosity (x)
|
|
2.376
|
2.532
|
2.844
|
2.988
|
y = 44.4 + 68.5x
|
| |
Inhibition (y)
|
0.00
|
82.6
|
87.1
|
94.1
|
96.7
|
g = 0.997
|
| Yanagimatsutake |
Viscosity (x)
|
|
1.944
|
2.112
|
2.088
|
2.424
|
y = -85.3 + 70.5x
|
| |
Inhibition (y)
|
0.00
|
51.5
|
54.2
|
70.8
|
86.1
|
g = 0.885
|
| *: m Pa.S
#: % of inhibition ! g : Coefficient
of correlation |
Effect of the dietary fibre fractions from Wakame
seaweed on apparent protein digestibility in vivo
Table 2. Body weight gain
and apparent digestibility of protein in young adult rats given
normal diets containing a 5% level of the soluble (SDF) or insoluble
(IDF) dietary fibre fraction separated from Wakame seaweed.
| Diet |
Wakame SDF
|
Wakame IDF
|
| Number of rats |
7
|
7
|
| Body weight gain
(g)a |
11.8± 4.8c
|
17.2± 4.0
|
| Total N intake
(mg)b |
655± 62
|
813± 125
|
| Faecal N excretion
(mg)b |
91± 29c
|
153± 23
|
| Protein N intake-A
(mg)b |
632± 65
|
669± 111
|
| Absorbed protein
N-B (mg)b |
564± 54
|
660± 113
|
| Apparent protein
digestibility-B/A x 100 (%)b |
89.2± 4.6c
|
98.6± 1.5
|
a) for 7 days. b) for the last 3 days. c) Significantly
lower than the IDF group by the student t-test (P < 0.01)
|
To test the relationship between the inhibition of
pepsin activity in vitro and the digestibility of dietary 1000 protein
in vivo, young adult rats were given a normal protein diet containing
the SDF or IDF from Wakame seaweed, as a representative of the seaweeds
and mushrooms used, for seven days. Apparent protein digestibility
for the last three days was determined. As shown in Table 2, both
nitrogen intake and faecal nitrogen excretion for the last three days
were larger in the IDF group than in the SDF group. There is a possibility
that the difference in the nitrogen intake derived from the IDF and
SDF might contribute to this, because the nitrogen content of the
IDF and SDF was different as described in the methods. Therefore,
the true nitrogen intake of dietary protein was estimated by subtracting
the nitrogen intake of SDF or IDF from the total nitrogen intake.
On the other hand, absorbed protein nitrogen was computed by subtracting
faecal nitrogen from total nitrogen intake. Subsequently, apparent
protein digestibility (%) was estimated by dividing absorbed protein
nitrogen by protein nitrogen intake.
As shown in Table 2, total nitrogen intake of the
SDF diet group tended to be smaller than that of the IDF diet group.
This is not due to the difference in preference for the diets but
to the difference in the nitrogen from the fibre fraction included
in the diet because protein nitrogen intakes estimated as above in
both groups were similar. On the other hand, absorbed nitrogen of
the SDF diet group tended to be smaller than that of the IDF diet
group. Consequently, rats given the SDF diet showed a significantly
lower protein digestibility, 89.2% (P<0.01) compared to rats given
the IDF diet, 98.6%. The value for the apparent casein digestibility
particularly in the IDF group seemed somewhat higher compared to the
data summarised by Gallaher et al.8. but Hove and King15
also reported that the fairly high apparent digestibility of casein,
95-96% when diets of 22% casein level with 0-5% levels of cellulose
were administered to rats. Though we did not determine here the digestibility
of casein in a diet with no fibre fraction since the present objective
was the comparison between the SDF and IDF from the Wakame seaweed,
the present animal experiment has been appropriately conducted to
see the difference between the SDF and IDF diets. The lowered digestibility
might be associated with the findings that a highly viscous poly-saccharide,
sodium alginate, which constitutes a greater part of soluble dietary
fibre in seaweeds, decreased the digestibility of protein in growing
rats6 and that soluble dietary fibres decreased trypsin
activity in vitro14. The lowered protein digestibility
by the SDF from the Wakame seaweed may have resulted in a significantly
lower body weight gain of the SDF group compared with that of the
IDF group (Table 2).
Conclusion
From the presen 1000 t results of the in vitro and
in vivo experiments, it can be concluded that soluble dietary fibres
with the viscous properties of seaweeds and mushrooms, which are different
from insoluble fibres, can lower the availability of dietary protein.
This finding may support the notion that soluble fibres in foods are
likely to decrease in protein utilisation since some other purified
soluble dietary fibres such as pectin and guar gum decreased not only
protein digestibility but also nitrogen retention in less amounts
than insoluble fibres8. The importance of this finding
in human nutrition is that we should take into consideration the low
availability of protein, particularly when dietary protein intake
is not adequate. Japanese people are known to eat several kinds of
seaweeds including the brown algae and their intakes for the past
twenty years are estimated to be about 5g per capita per day16.
The daily intake of soluble and insoluble fibres from seaweeds might
be presumed to be 1.25g per capita per day when the amounts are computed
by our previous data1 on the assumption that the contents
of both total dietary fibre and soluble dietary fibre as a percentage
of total dietary fibre would be approximately 50%. However, since
mushroom consumption by the Japanese is estimated to be about 10g
per capita per day in 199116 the dietary fibre content
would be about 0.18g for soluble fibre and 2.82g for insoluble fibre.
Thus, the total intake from seaweeds and mushrooms account for l.43g
of soluble fibre and 4.07g of insoluble fibre. Of the total dietary
fibre intake of 17.33g per capita per dayl7, the soluble
fibre represents 8.25% while the insoluble represents 23.48%. To what
extent such fibre intakes can affect dietary protein availability
is a problem that needs further study.
References
- Y Horie, K Sugase, and K Horie. Dietary fibre in
brown algae and mushrooms. ASEAN Food J 1991; 6: 32-33.
- L Prosky, N-G Asp, I Furda, JW DeVries, TF Schweizer,
and BF Harland, Determination of total dietary fiber in foods and
food products. Collaborative study. J Assoc Of Anal Chem; 1985;
68: 677-679.
- WJL Chen, and JW Anderson. Hypocholesterolemic
effects of soluble fibers . In: GV Vahouny and D Krichevsky eds.
Dietary fiber-basic clinical aspects, Plenum Press1986; 275-286.
- TA Miettinen. Dietary fiber and lipids. Am J Clin
Nutr 1987; 45: 1237-1242.
- Y Watanabe, K Kawai, and K Yamashita. Effect of
a dietary fiber-protein mixture on glucose tolerance and lipid metabolism
in diabetics. J Jpn Soc Nutr Food Sci(in Japanese) 1990; 43: 103-107.
- S Ikegami, F Tsuchihashi, H Harada, N Tsuchihashi,
E Nishide, and S Innami. Effect of viscous indigestible polysaccharides
on pancreatic-bilinary secretion and digestive organs in rats. J
Nutr 1990; 120: 353-360.
- JC Houck, J Bhayana, and T Lee. The inhibition
of pepsin and peptic ulcers. Gastroenterology 1960; 39: 196-200.
- B0 Schneeman, and D Gallaher. Effects of dietary
fiber on digestive enzymes. In:GA Spiller, and D Chen. eds. CRC
Handbook of dietary fiber in human nutrition, CRC Press Inc, Florida,
1986; 305-312.
- FM Larsen, N Wilson,. and PJ Moughan, Dietary fiber
viscosity and amino acid digestibility, proteolytic digestive enzyme
activity and digestive organ weights in growing rats. J Nutr 1994;
124: 833-841.
- S Kurasawa, T Sugahara, and J Hayashi. Determination
of dietary fib c33 er in chitin-containing mushrooms by the enzymatic-gravimetric
method. J Jpn Soc Nutr Food Sci (in Japanese) 1991; 44: 293-303.
- ML Anson. The estimation of pepsin, trypsin, papain
and cathepsin with hemoglobin. J Gen Physiol 1938; 22:7 9-89.
- Y Horie, and K Sugase. Effects of some brown algae
and mushrooms on protein digestion by pepsin in vitro. J Nagoya
Municipal Women's Coll (in Japanese) 1991; 47: 31-37.
- Y Horie, and K Ashida, Stimulation of hepatic protein
synthesis in rats fed an adequate protein diet after a low protein
diet. J Nutr 1971; 101: 1319-26.
- SK Dutta, and J Hlasko. Dietary fiber in pancreatic
disease: Effect of high fiber diet on fat malabsorption in pancreatic
insufficiency and in vitro study of the interaction of dietary fiber
with pancreatic enzymes. Am J Clin Nutr 1985; 41: 517-525.
- EL Hove, and S King. Effects of pectin and cellulose
on growth, feed efficiency, and protein utilization, and their contribution
to energy requirement and cecal VFA in rats. J Nutr 1979; 109:1274-78.
- Ministry of Health and Welfare of the Japanese
Government. The 1991 present national situations of nutrition in
Japan (in Japanese), Daiichi Shuppan, Tokyo, 1993;65. Snow Brand
Institute, Tokyo, 1994; No.17, 5.
- Japan Conference of Local Hygiene Institute. The
dietary fiber composition table (in Japanese), Daiichi Shuppan,
Tokyo, 1990; 45.
Physiological differences of soluble
and insoluble dietary fiber fractions of brown algae and mushrooms
in pepsin activity in vitro and
protein digestibility
Y Horie, K Sugase, K Horie
Asia Pacific Journal of Clinical
Nutrution (1995) Volume 4, Number 2: 251-255



Copyright © 1995 [Asia Pacific Journal of Clinical Nutrition]. All
rights reserved.
Revised:
January 19, 1999
.
 to the top
to the top
0