Asia Pacific J Clin Nutr (1995) 4: 35-38

Body composition measurement in
normal children: ethical and methodological limitations
Louise A Baur BSc(Med), PhD,
FRACP
Department of Medicine (Endocrinology), University of Sydney,
NSW, Australia.
There are significant ethical. practical and theoretical
issues that need to be considered when measuring body composition
in normal children. For example, when evaluating the use of techniques
that involve ionizing radiation, then the benefit to the volunteer
subject, or society at large, needs to be balanced against the likely
harm to the subject. For children, the detriment per unit dose may
be two to three times larger than that for young adults. At present
the decision as to the acceptable radiation dose limit for healthy
children undergoing research studies remains debatable. Most techniques
for measuring body composition require specific validation of their
precision and accuracy when used with small subjects; adaptation
of existing methods may thus be necessary in order to measure children.
In addition, techniques such as densitometry and dual energy X-ray
absorptiometry may be impractical for use in young children. A major
theoretical issue to be considered is that most body composition
techniques assume a constant density or chemical composition of
the fat-free mass (FFM). However, the FFM in children does not consist
of fixed proportions of water, protein and mineral; rather, the
proportions of these change during growth, with water content decreasing
and protein and mineral content increasing. Caution must therefore
be used in the application of adult-derived body composition constants
and equations to children.
Introduction
Accurate measurement of body composition in healthy
children is important for a number of reasons. Firstly, it enables
a better understanding of the major changes in chemical composition
of the body that occur as a normal part of growth and development.
In so doing, the relationship between easily measured growth parameters
and such variables as body protein, potassium and bone mineral content
can be determined1. Furthermore, measurement of body composition
in normal children provides control data which are vital for the interpretation
of body composition measurements in children with varying disease
processes. However, there are significant ethical, practical and theoretical
issues that need to be considered when measuring body composition
in healthy children.
Ethical issues
In all clinical research, especially that involving
normal subjects, it is vital to consider the benefit that is to be
derived from a particular study and to balance this against the possible
risks to the subject2. These risks include those arising
from the use of ionizing radiation as well as from blood sampling.
Ionizing radiation
Several methods for determining body composition involve
the use of ionizing radiation including dual-energy X-ray absorptiometry
(DEXA)3, neutron capture analysis4 and computed
tomography5. Can any of these techniques be used in assessing
children? Are there any established principles that can be used in
evaluating the ethical use of ionizing radiation in normal children?
The International Commission on Radiological Protection
(ICRP) recently published its report on Radiological Protection in
Biomedical Research2 in which established principles relating
to the use of ionizing radiation in research were highlighted6.
These principles are meant to be applied to all volunteers for medical
research, including children. Firstly, it is important to consider
why the study is needed; this includes an assessment of the benefit
that will result from the study, the extent to which that benefit
is to the volunteer or to society at large, and the type of benefit
that is likely to arise. Secondly, it is necessary to assess the likely
harm to the volunteer from the study.
The biological effects of ionizing radiation can be
classified into two categories: deterministic and stochastic7.
Deterministic effects result from cell or tissue death and are characterized
by a severity that increases with dose above a certain clinical threshold.
They include such clinical findings as lens opacities, hair loss and
reduced fertility. However, a key feature of a deterministic effect
is that the dose threshold is well-defined and so can be avoided in
biological research. In contrast, stochastic effects result from radiation-induced
changes in cell nuclei and lead to cancer induction or to genetic
damage (evident only in the next generation). They are effects for
which the probability of an occurrence, rather than its severity,
is regarded as a function of dose.
Furthermore, there is an apparent absence of a dose
threshold for a stochastic effect; in effect, radiation exposure at
any dose results in a probability of induction of harm. It is this
biological effect which needs to be considered in any use of ionizing
radiation in normal subjects. The risk resulting from the use of ionizing
radiation is therefore equivalent to the sum of the probability of
fatal cancers caused by the radiation, plus the weighted probability
of non-fatal cancers, plus the probability over all succeeding generations
of serious hereditary disease resulting from the dose. Of additional
specific interest is the fact that at young ages the detriment for
a given radiation dose is higher ('2 to 3 times larger') than for
young adults2.
However, before the biological effects of ionizing
radiation from a given procedure can be estimated, a reliable assessment
of the dose is necessary2. This needs to take into account
specific age and sex risk factors for different organs8.
Assessment of dose rates for small subjects is therefore particularly
necessary; simple extrapolation from dosimetry estimates for adults
will usually be inappropriate. This is very important when using body
composition techniques, most of which were initially developed for
use in adults4,9.
The ICRP2 has modified the original WHO
classification of research projects6 into categories depending
on the amount of radiation dose to be received by the subject. Essentially,
the categories are defined by the level of risk, and the boundaries
between categories may be equated to a level of dose. The lowest risk
category (Category I) is of the order of 10-6 or less (ie
regarded as trivial) and has a corresponding effective dose range
of <0.1 mSv (equivalent to a few weeks of background radiation).
The level of benefit needed to justify radiation exposure at this
level would be only minor. Perhaps it is in this risk category that
studies on children can be performed. The highest risk category (Category
III) includes risks of approximately 10-3 (ie a moderate
risk) corresponding to an effective dose range of >10 mSv (for
comparison, the current annual dose limit for occupational exposure
is 20 mSv per year); a substantial level of societal benefit would
be required to justify the risks with such a dose. Risk Category II
lies between these two extremes of risk and requires an intermediate
to moderate level of societal benefit for justification of exposure.
The principles stated above can be applied in assessing
the ethical use of ionizing radiation in normal children. It is noteworthy
that the ICRP does not specify any particular dose limit for children;
strict recommendations as to which body composition techniques may
or may not be used cannot therefore be given. In essence, the risks
should be minimal and the information sought should not be able to
be obtained by other means.
Blood sampling
Another ethical issue to be considered in the measurement
of body composition in children is blood sampling. Venous access,
particularly in well-nourished young children, may be difficult, with
the potential for the procedure to be psychologically distressing
to both the child and the parent. It is therefore important to firstly
consider whether blood sampling is required. For example, collection
of urine or saliva samples, rather than blood, would be preferable
when estimating deuterium-space in children10. However,
if blood specimens are required for a study (eg measurement of bromide
space11) then they need to be collected skillfully. Finger-stick
sampling, performed expertly, may be less worrying than a venepuncture
although this is not necessarily the case. Furthermore, local anaesthetic
creams, applied topically prior to the venepuncture, can minimize
much potential
Practical issues in measuring body composition
in children
Practical recommendations
Some methods of assessing body composition that are
readily used in adults are inappropriate for the assessment of children
from a practical point of view. For example, young children are unable
to lie still for extended periods of time and thus techniques in which
this is a requirement, such as DEXA3, will be unsuitable
for many children in this age range. In practical terms, children
below the age of four or five years are rarely suitable as DEXA subjects,
except for those under a few months of age who may sleep or lie quietly
through the assessment. Measurement of total body potassium by the
counting of 40K gamma rays may also be difficult with some
facilities. The use of whole body counters which require the subject
to lie quietly in a small, lead-lined room is obviously inappropriate
for young children. More 'subject-friendly' counters in which the
subject is visible at all times have been used for assessment of babies
through to adults12; such facilities will, however, have
higher background levels of radiation. This problem of age-appropriate
assessment is also seen with densitometry13. Clearly only
those who are water-confident can be assessed with this technique;
children under the age of six or seven years are therefore not usually
suitable as subjects.
Another practical issue relates to the administration
of young children of stable isotopes, such as deuterium or 18O,
or other tracer substances. Accurate dosing can be challenging in
young children who may refuse to drink the tracer, or even spit it
out after it has been given! For this reason it is often preferable
for an experienced investigator to carefully administer the tracer—in
the case of babies, with a fine tube attached to a syringe or, in
older infants and toddlers, with a feeding cup10. It is
also essential to recognize honestly when the tracer has been spilled
and to stop the study at that stage without fruitlessly proceeding.
Collection of physiological fluid samples can also
be difficult. However, urine bags can be used in babies and toddlers,
although these may leak, slip off or become contaminated with faeces.
In addition, saliva samples can be collected from infants by swabbing
the mouth with a small sponge or absorbent cotton wool10.
Finally, during any assessment of body composition
it is important to ensure that the child remains calm, content and
reasonably cooperative. This is most likely to happen if the parents
are involved and understand the requirements of the study, if the
study takes place in a pleasant environment and if a reassuring and
sympathetic approach is used.
Specific validation of techniques for small
subjects
Another set of methodological problems is related
to the need for specific validation of many techniques for their use
in small subjects. For example, in the measurement of nitrogen gamma
ray emission with the technique of neutron capture analysis, the smaller
the subject then the greater the relative contribution of background
counts to the total number of gamma rays measured4. The
precision of the measurement is therefore likely to be worse in a
small subject than it would be in a bigger subject4. In
fact, many methods which are used in adult body composition measurements
may need to be modified and specifically validated if they are to
be used in children.
Theoretical issues
There are also major theoretical issues to be considered
when measuring body composition, even in normal children. It is vital
to understand the assumptions that underlie many techniques, and any
specific problems associated with their use. Fundamental to many body
composition techniques is the assumption that the fat-free mass (FFM)
is of constant density and chemical composition.
The concept of chemical maturity was first introduced
in 1923 by Moulton14. He defined it as 'The point at which
the concentration of water, proteins and salts becomes comparatively
constant in the fat-free cell. . .' Moulton suggested that mammals
reach chemical maturity at approximately 4-5% of their total life
span, and proposed this to be at about 4 years of age in humans. At
chemical maturation the FFM was estimated to be 70-75% water, 18-20%
protein and 5-9% mineral ('ash'). However, Moulton's conclusions were
based upon observations of a limited number of carcass analyses in
foetuses, infants and adults.
Detailed calculations of the body composition of the
growing child and adolescent were first published by Fomon, Haschke
and co-workers in the early 1980s15,16. These were based
upon estimates of total body water, total body potassium and total
body calcium (from single-photon absorptiometry) obtained from both
their own work and a review of the literature15,16. Using
these data and a number of assumptions, the body composition of 'reference
children' at different ages was able to be determined. These estimates
allowed the change in the composition of the FFM with growth to be
assessed. Fomon and co-workers15,17 estimated that the
FFM of the term infant is 80.6% water (61% of the water mass being
extracellular), 15.0% protein and 3.7% mineral. Subsequently there
is a decrease in the percentage of water, an increase in the proportion
of intracellular to extracellular water and an increase in the percentage
of protein. Increasing mineralization of the FFM also occurs, particularly
during adolescence. The composition of the FFM in the adult male quoted
by Haschke16 consists of 72.6% water (43% of the water
mass is extracellular), 20. 1% protein and 6.7% mineral, this being
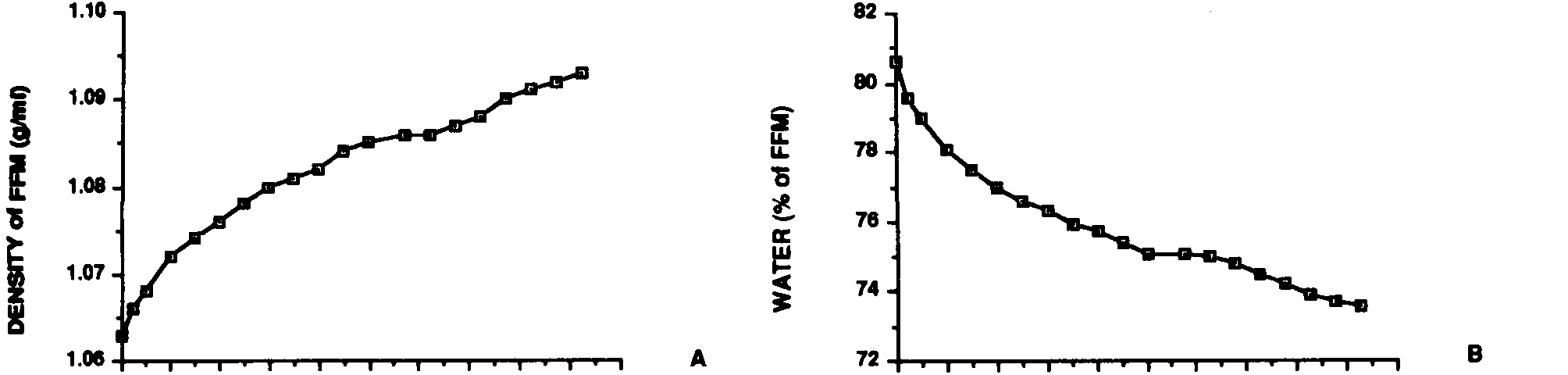
a significant change from the FFM composition of the infant. Figure
1 is a graphical representation of these changes in the density of
the FFM (calculated from the chemical constituents), and the water
content of the FFM, based upon the data of Fomon et al.15
and Haschke16 for male children and adolescents. Other
workers have found similar changes in density and body water content
by employing different techniques18,19. In addition we
have demonstrated, using direct measurements of total body nitrogen
in normal children, that the protein content of the FFM increases
with age20. Such findings confirm that chemical maturity
of the FFM does not occur until at least puberty.
| Figure
1. Changes in the density (A) and water content (B) of
the fat-free mass (FFM) from birth to 18.5 years in the male reference
child and adolescent. Data are those of Fomon et al.15
and Haschke16. These studies show that chemical maturity
does not occur until late puberty. |
 |
The concept of chemical maturity has great significance
for the measurement of body composition in childhood and adolescence;
this aspect was reviewed in detail by Lohman in 198619.
It is essential to recognize that the assumption of constant density
or of constant water, protein or mineral content is often routinely
used in the estimation of FFM by such techniques as densitometry,
deuterium-space analysis, gamma ray spectrometry (for measurement
of total body potassium), skinfold thickness measurement, bioelectrical
impedance analysis and DEXA13. In the usual analysis of
data obtained using these techniques, adult-derived constants are
used. However, it may be appropriate to modify these basic assumptions,
for example by using age- and sex-adjusted constants19.
Finally, the use of more than one method to assess to body composition
in children has the potential to allow a more precise and accurate
assessment to be performed. There are many models of body composition,
from the basic two-compartment model of fat mass and FFM, which tends
to be the one most commonly used in paediatric measurements, to more
complex models, usually derived from the use of two or more different
techniques. Multi-compartment models are increasingly used in adult
body composition measurements21; their use in the assessment
of children may allow body composition to be determined by means that
are less dependent on non-validated assumptions.
Conclusion
Therefore, in measuring body composition in normal
children a number of factors need to be recognized. The major ethical
issue to be considered relates to the balance of the level of benefit
to be derived from the study versus the level of risk to which the
subject may be exposed from the study. When dealing with children
these risks should be minimal. While ionizing radiation may be a significant
factor with some methods for measuring body composition in childhood,
it should be realized that there other forms of risk such as those
associated with blood sampling. A number of practical issues should
also be recognized. For example, some techniques for measuring body
composition may be inappropriate for use in small children while others
may require specific validation in order to be used for the accurate
and precise assessment of small subjects. Finally, many body composition
techniques assume a constant density or chemical composition of the
FFM, an assumption that is not valid in children. Caution must therefore
be used in the application of adult-derived body composition constants
and equations to children.
References
- FAO/WHO/UNU. Energy and protein requirements. WHO
Tech 724. Geneva: WHO, 1985.
- International Commission on Radiological Protection.
Radio- 14 logical protection in biomedical research. ICRP Publication
62. Ann ICRP 1991; 22(3). 15
- Mazess RB, Barden HS, Bisek IP, Hanson J. Dual-energy
Xray absorptiometry for total-body and regional bone-mineral and
soft-tissue composition. Am J Clin Nutr 1990; 51: 161106 12.
- Baur LA, Allen BJ, Rose A, Blagojevic N, Gaskin
KJ. A total body nitrogen facility for paediatric use. Phys Med
Biol 1991; 36: 1363-75. 17
- Kvist H, Sjostrom L, Chowdhury B, Alpsten M, Arvidsson
B, Larsson L, Cederblad A. Body fat and adipose tissue determinations
by computed tomography and by measurements of total body potassium.
In: Yasumura S, Harrison JE, McNeill KG, Woodhead AD, Dilmanian
FA, eds. In vivo body composition 19 studies. Recent advances. New
York: Plenum, 1990: 197-218.
- WHO. Use of ionizing radiation and radionuclides
on human beings for medical research, training and non-medical purposes.
WHO Tech Rep Ser 611. Geneva: WHO,1977.
- International Commission on Radiological Protection.
Recommendations ICRP Publication 60. Ann ICRP 1991; 21 (1-3).
- Land CE, Sinclair WK. The relative contributions
of different 21 organ sites to the total cancer mortality associated
with lowdose radiation exposures. Ann ICRP 1991; 22 ( I ): 33-57.
- Allen BJ, Bailey GM, McGregor BJ. Dose equivalent
distribu tions in the AAEC total body nitrogen facility. In: Proceedings
of the fourth Australian conference on nuclear techniques of analysis.
Lucas Heights, Sydney: AINSE,1985: 101-3.
- Prentice AM, Coward WA, Cole TJ, Schoeller DA,
Haggarty PA. Practical recommendations and worked examples. In:
Prentice AM, ed. The doubly-labelled water method for measuring
energy expenditure. A consensus report by the IDECG Working Group.
Vienna: IAEA,1990: 212-247.
- Vaisman N, Pencharz PB, Koren G, Johnson JK. Comparison
of oral and intravenous administration of sodium bromide for extracellular
water measurements. Am J Clin Nutr 1987; 46: 1 4.
- Shepherd RW, Holt TL, Greer R, Cleghorn GJ, Thomas
BJ. Total body potassium in cystic fibrosis. J Pediatr Gastroenterol
Nutr 1989; 9: 200-5.
- Lukaski HC. Methods for the assessment of human
body composition: traditional and new. Am J Clin Nutr 1987; 46:
537-56.
- Moulton CR. Age and chemical development in mammals.
J Biol Chem 1923; 57: 79-97.
- Fomon SJ, Hschke F, Ziegler EE, Nelson SE. Body
composition of reference children from birth to age 10 years. Am
J Clin Nutr 1982; 35: 1169-75.
- Haschke F. Body composition of adolescent males.
Part 1. Total body water in normal adolescent males. Part 11. Body
composition of the male reference adolescent. Acta Paediatr Scand
1983; (Suppl)307: 1-23.
- Fomon SJ. Body composition of the male reference
infant during the first year of life. Pediatrics 1967; 40: 863-70.
- Boileau RA, Lohman TG, Slaughter MH, Ball TE, Going
SB, Hendrix MK. Hydration of the fat-free body in children during
maturation. Hum Biol 1984; 56: 651 66.
- Lohman TG. Applicability of body composition techniques
and constants for children and youths. Exerc Sport Sci Rev 1986;
14: 325-57.
- Baur LA, Allen JR, Waters DL, Gaskin KJ. Total
body nitrogen in prepubertal children. In: Ellis KJ, Eastman JD,
eds. Human body composition: In vivo methods, models and assessment.
NewYork:PlenumPress,1993: 139-42.
- Heymsfield SB, Waki M. Body composition in humans:
advances in the development of multi-compartment chemical models.
NutrRev 1991;49:97-108.

Copyright © 1995 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.