1000
Asia Pacific J Clin Nutr (1995) 4: 187-189
Asia Pacific J Clin Nutr (1995) 4: 187-189

Whole body measurement of C, N
and O using 14 MeV neutrons and the associated particle time-of-flight
technique
S. Mitra1,
J. E. Wolff1, R. Garrett2
and C. W. Peters3
- Growth Physiology Group, AgResearch,
Ruakura, Hamilton, New Zealand;
- Department of Physics, University
of Auckland, Auckland, New Zealand;
- Nuclear Diagnostic Systems Inc.,
Springfield, VA, USA.
Our aim has been to construct a portable prototype
instrument for measuring the whole body composition in vivo of growing
lambs in terms of fat. protein and water by determining the mass
of carbon, nitrogen and oxygen present. A small and compact sealed
tube neutron generator which has the capability of exploiting the
associated particle time-of-flight technique has been used for prompt
gamma 14 MeV neutron activation analysis of C, N and O. This technique
allows only gamma rays generated by neutron reactions within a defined
volume to be recorded and offers a superior signal-to-noise ratio
over existing prompt gamma neutron activation techniques. Based
on the results obtained from irradiating a 41.4 kg meat phantom,
we anticipate that an instrument comprising the neutron generator
and four 15 x 15 x 45 cm Nal(TI) gamma ray detectors can be assembled
to determine in vivo, protein, fat and water with precisions of
4.1, 5.4 and 1.2% (CV), respectively, within a 15 min scan. The
radiation dose delivered would be ~0.03 mSv.
Introduction
Given the fixed stoichiometric proportions of N, C
and O in protein, fat and water, proximate composition of the body
can be derived from its elemental analysis. Despite use of in vivo
neutron activation analysis for more than two decades to measure elemental
composition of the human body1-3, there is not yet an instrument
which can measure N, C, and O during a single irradiation and detection
procedure.
Instruments constructed during the 1980s have mainly
used prompt gamma emissions to detect either N from the 10.83 MeV
radiative capture emission1 or in a few instances, C from
the inelastic scattering reactions with 14 MeV neutrons4,5.
Kehayias et al.5 proposed that O be measured simultaneously.
The major problems encountered have been the interferences and poor
signal-to-noise ratios that result from scattered neutrons, and relatively
high radiation doses.
We6 and Hollas et al.7 demonstrated
the feasibility of using a coincidence system between the associated
alpha particle and the 14 MeV neutron induced inelastic gamma ray
to improve the signal-to-noise ratio so that N, C and O can be determined
in large biological samples with good precision. Briefly, the alpha
1000 particle associated with a 14 MeV neutron produced by the 3H(d,n)4He
reaction in the neutron generator is emitted in the opposite direction.
Its detection specifies the neutrons in a given solid angle and, by
appropriately gating the gamma detector, the resulting gamma ray spectrum
is derived predominantly from neutron reactions within the defined
volume of the sample.
A compact associated particle, sealed tube, neutron
generator (APSTNG) with an internal alpha detector is now commercially
available. Its success in detecting contraband drugs or explosives
and use in coal analysis8 (where the elements of interest
are similar to those for body composition) has been demonstrated.
Here we report first results for measuring the C, N and O content
of a large meat sample.
Methods
The
APSTNG
The APSTNG and its high voltage control system was
supplied by Nuclear Diagnostic Systems Inc., USA. It contained a mixture
of deuterium and tritium, that was ionized by a Penning ion source,
accelerated by a potential of 95 kV between the ion source cathode
and target and focused to a spot (~1 mm diameter) on the target to
produce neutrons and alpha particles by the 3H(d,n)4He
reaction. Ion beam current (typically, 1 m A to obtain 106 n/s) was controlled by heating a getter into
which the 2H and 3H were absorbed. The tube
also housed an alpha particle detector (3.7 cm ø ZnS screen positioned
4.5 cm from the target) which was interfaced to a Hamamatsu R580-15
fast rise time photomultiplier.
Data
acquisition
Anode signals from both the alpha detector and a 12.5
cm diameter x 10 cm Bicron Nal(TI) gamma detector were each fed to
timing filter amplifiers and their outputs to constant fraction discriminators.
For alpha pulses, the discriminator was set just above the photomultiplier
dark current with the APSTNG off while for gammas, the discriminator
was set to reject 0.5 MeV pulses and below. Resulting logic pulses
from alpha and gamma detectors, respectively, started and stopped
the time-to-amplitude converter (TAC). Its output, the timeof-flight
spectrum, was recorded with an analog to digital converter (ADC) and
multichannel analyzer (MCA). The full width half maximum portion,
corresponding to the time when most prompt gamma rays were emitted
from a sample within the neutron beam, was selected by a single channel
analyzer (SCA). The SCA output triggered a linear gate which allowed
only gamma-ray energies within the defined time window to be recorded
by an MCA and stored in a computer for subsequent spectral analysis.
Phantom
construction
A 38.2 kg calibration phantom containing physiological
amounts of the major body elements (ICRP 1975)9 was constructed.
Its elemental composition by weight was 8.5% H, 18.2% C, 68.1% O,
2.7% N, 1.08% Ca, 1.02% P, 0.19% S, 0.17% K, 0.06% Na and 0.1% Cl.
The solution was contained in polyethylene tubes (5 cm diameter x
80 cm) with a wall thickness of 0.15 mm and heat sealed. These were
bundled into a cylindrical phantom (25 cm diameter by 80 cm) and wrapped
with polyethylene sheeting. For a meat phantom of 41.4 kg we filled
the same type of tubes with minced meat.
Irradiation
and counting
The calibration and meat phantoms were each irradiated
for 1 hour in scanning mode at a distance of 30 cm f 1000 rom the
APSTNG. At this distance the neutron 'beam', defined by the associated
alpha particle, was also 30 cm in diameter. The detector was positioned
above the phantom and outside the defined neutron 'beam' with its
face being 29 cm from the beam axis and perpendicular to it. The detector
was shielded from direct neutrons by housing it in a 5 cm thick lead
cave with an outer, 15 cm thick, layer of wax and borated polystyrene.
The total integrated counts in a region of interest
(ROI) for the 1 hour irradiation were normalized to a constant neutron
flux based on the recorded number of alpha counts. From this information,
and assuming that sections of sample outside of the defined beam were
shielded, the radiation dose during a 1 hour scan was calculated to
be 0.12 mSv10.
Spectral
analysis
Prompt gamma rays of interest from the fast neutron
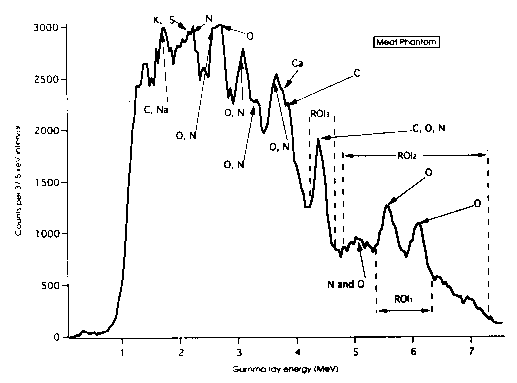
reactions: C; 4.43 MeV, N; 7.03, 5.1, 5.03 and 4.46 MeV and O; 7.12,
6.92, 6.13 and 4.43 MeV. Other intense gamma rays below 4 MeV are
also detected but these suffer interferences from Ca, Cl, K and S
(see Fig. 1). Consequently for the present report we have used only
counts from the 4-7.5 MeV regions where the only elemental interferences
are between C, N and O. Following the algorithm developed by Peters11,
ROI1 was set at 5.35-6.25 MeV, ROI2 was 4.85-7.28
MeV and ROI3 was 4.22-4.68 MeV. Using liquid N2
and H2O we recorded pure spectra due to N and O, respectively,
to define the interference coefficients. Background counts due to
random coincidence events and from neutrons scattered from the sample
into the detector within the defined time window were measured as
follows: for ROI1 and ROI2 background counts
were obtained from wax scatterers as there are no gamma rays from
C above 4.68 MeV while for ROI3, net counts were obtained
by peak stripping from the underlying continuum.
Figure 1. The g spectrum of the meat phantom obtained within
a 25 ns time window.
Results and
discussion
In Table 1 we present estimates for radiation dose
and the precision for measuring N, C and O in the meat phantom when
compared with other detector geometries. Estimates for the four detector
system for N utilizing the (n,g ) reaction are derived by scanning
the same meat phantom at the Auckland Hospital, Body Composition Facility
which uses two 238Pu/Be neutron sources and four 12.5 cm
diameter by 15 cm Nal(TI) gamma ray detectors12. The six
detector system (see Table 1, third line) scales our results, obtained
using the APSTNG and a single detector, to the same detector volume
and scanning time as used at Auckland Hospital. The results show that
precision for estimating N is comparable while radiation dose to the
subject would be substantially reduced.
Table 1. Dose and precision of measuring N,
O and C in a 41.4 kg phantom of minced meat with different NaI detector
configurations.
| |
|
|
Precision, CV%, (n=10) |
| Number of 1000 detectors
|
Irradiation time |
Dose msv |
N |
O |
C |
| ONE (12.5cm ø x 10cm) |
1 hour |
0.12 |
11.5 |
2.3 |
9.2 |
| FOUR (Auckland Hospital
12.5cm ø x 15cm) |
30 min |
0.2 |
5.0 |
-- |
-- |
| SIX |
30 min |
0.06 |
6.7 |
1.4 |
5.4 |
| FOUR (15 x 15 x 45cm) |
15 min |
0.03 |
4.1 |
<1 |
3.3 |
In order to obtain an acceptable precision for the
measurement of body composition in farm animals, however, further
enhancements of detection efficiency are required. In the fourth line
of the table we have scaled our results from a one detector system
to those which can be expected from the use of four large (15 cm x
15 cm x 45 cm) NaI detectors. These detectors are becoming available
commercially and are obviously needed before the APSTNG system can
achieve its full potential.
In Table 2 we compare analyses of meat composition
by use of the APSTNG with existing procedures for proximate composition.
The C, N and O content of the meat phantom was derived from the ratios
of its elemental count rates with those obtained from the calibration
phantom. Mass of protein was 6.25 x N while fat and water were determined
from a four compartment model of body composition comprising protein,
fat, water and minerals and their fractional content of C, N and O9.
For this model, the small glycogen compartment has been ignored while
5% of phantom weight has been assumed for the mineral compartment9.
Propagation errors that a 1000 rise from errors in the counting of
each element are shown in Table 2. It can be seen that there is excellent
agreement between the two methods of analysis for fat and water 2
while we have a significant discrepancy for protein. Reasons for this
are being examined.
Table 2. Measured content of protein, fat and
water in the meat sample.
| Component |
NAA (14MeV) kg |
% |
Chemical analysis % |
| Protein |
6.0± 0.3 (4.1 %) |
14.5± 0.6 |
16.5± 0.4 |
| Fat |
7.84± 0.42 (5.4%) |
18.95+1.02 |
19.0± 0.8 |
| Water |
24.66± 0.29 (1.2%) |
59.6± 0.7 |
59.7± 0.9 |
| Sum |
|
93.05 |
95.2 |
| Ash |
|
|
4.55± 0.57 |
Conclusion
A transportable instrument based upon the APSTNG and
four 15 x 15 x 45 cm NaI(TI) detectors can be assembled for measuring
the body composition of humans or farm animals in vivo. It would provide
a simultaneous measure of protein fat and water to good precision
within a 15 minute scanning time. The radiation dose (~0.03 mSv) would
be almost one-tenth of the dose from existing facilities.
Acknowledgments—This work has been financially
sup ported by the NZ Foundation for Research Science and Technology.
Supply of the APSTNG and its High Voltage Control d56 System by Nuclear
Diagnostic Systems (USA) at cost price and loan of the nuclear electronics
by the Physics Department, University of Auckland is appreciated.
We thank Professor G L. Hill and Dr L. D. Plank for use of the Auckland
Hospital Body Composition facility.
References
- Beddoe AH, Hill GL. Clinical measurement of Body
Composition using in-vivo neutron activation analysis. J Parenteral
Enteral Nutr 1985; 9: 504-520.
- Cohn SH, Parr RM. Nuclear based techniques for
the in-vivo study of human body composition. Clin Phys Physiol Meas
1985; 6: 275-301.
- Chettle DR, Fremlin JH. Techniques of in-vivo neutron
activation analysis. Phys Med Biol 1984; 29: 1011-1043.
- Kyere K, Oldroyd B, Oxby CB, Burkinshaw L, Ellis
RE, Hill GL The feasibility of measuring total body carbon by counting
neutron inelastic scatter gamma rays. Phys Med Biol 1982; 27: 805-817.
- Kehayias JJ, Zhuang H. Use of the Zetatron D-T
neutron generator for the simultaneous measurement of carbon, oxygen
and hydrogen in vivo in humans. Nucl Instr and Methods 1993; B79:
555-559.
- Garrett R, Mitra S. A feasibility study of in vivo
14-MeV neutron activation analysis using the associated particle
technique. Med Phys 1991; 18: 916-920.
- Hollas CL, Ussery LE, Butterfield KB, Morgado RE.
A method for in vivo determination of carbon and oxygen using prompt
gamma radiations induced by 14.7 MeV neutrons. In: Yasumura S et
al., eds. Advances in in vivo Body Composition Studies. New
York: Plenum,1990: 395-400.
- Gordon CM, Peters CW. A fast-neutron Probe for
Tomography and Bulk Analysis. Int J Radiat Appl Instrum Part A 1990;
41: 1111-1116.
- ICRP. Report of the Task Group on Reference Man.
ICRP Report 23. Oxford; Pergamon, 1975: 289-327.
- Goussev NG. Relationship between Dose Equivalent
(Absorbed Dose) and Fluence (Flux Density). In: Jaeger IRG, ed.
Engineering Compendium on Radiation Shielding, New York: Springer-Verlag,
1968; 1: 12.
- Peters CW. Unpublished work.
- Sutcliffe JF, Mitra S, Hill GL. In vivo measurement
of total body carbon using 238Pu/Be neutron sources. Phys Med Biol
1990;35: 1089-1098.

Copyright © 1996 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
 to the top
to the top
0