1000
Asia Pacific J Clin Nutr (1995) 4: 105-108
Asia Pacific J Clin Nutr (1995)
4: 105-108

Body composition of HIV-infected
male adults with wasting syndrome
KJ Ellis*, RJ Shypailo, JM Pivarnik, BH
Jenks, P Walzel and PDK Lee
Department of Pediatrics, USDA/ARS Children's Nutrition
Research Centre, Baylor College of Medicine, Houston, Texas, USA;
*Texas Children 's Hospital, Houston, Texas,
USA.
Chronic weight loss is a common characteristic
of HIV infection; its full etiology remains unknown. We report
body composition measurements for 39 adult males with wt loss
³ 10% or a body mass index (BMI) below
19.8 kg • m-2 while receiving stable antiretroviral
therapy, and no recent history of opportunistic infection, malignancy,
Kaposi sarcoma, or therapy with anabolic agents. CD4+ counts ranged
from 2 to 531; 30 subjects having counts £ 200. Body composition was
measured by 40K counting, dual-energy X-ray absorptiometry
(DXA), and anthropometry. The reference body composition measures
were total body potassium (TBK), lean tissue mass (LEAN), fat
mass (FAT), and percentage body fat (%FAT). In addition, nutritional
assessment was based on a 2-d food diary. The mean TBK was 90.2%
± 10.8% of normal controls, while
the %FAT averaged only 14.4% ± 5.3%, also below the normal
range. Reasonable estimates of these body composition compartments
were obtained using a combination of BMI, mid-arm circumference
(MAC), and triceps skinfold measurements (TSF).
Introduction
Progressive weight loss is common in the human immunodeficiency
virus (HlV)-positive individual1,2. Many factors appear
to contribute to the chronic wasting, such as diminished and nutritionally
inadequate food intake, intestinal dysfunction including malabsorption,
and altered metabolism3. This weight loss, believed to
increase morbidity for the AIDS patient, is sufficiently common; the
US Centres for Disease Control have catergorized a wt loss > 10%
as 'HIV wasting syndrome.' Although various anthropometric measurements
are available, their accuracy for body composition assessment in this
instance are unknown. The use of body weight alone or in conjunction
with height to provide body mass index (BMI), are not generally recognized
as good quantitative indicators of nutritional status in malnourished
individuals. Furthermore, only a few studies have examined in detail
the alterations in body composition associated with HIV or the HIV
wasting syndrome. Therefore, we wanted to determine if anthropometry
could provide an appropriate alternate assessment of body composition
for this population when it is the only technology available.
Methods
Subjects
All studies performed were approved by Institutional
Review Boards and informed consent was obtained from each subject,
who was referred by the primary physician or recruited through specific
advertisements. All subjects were clinically stable and had been receiving
antiretroviral therapy (AZT, ddC, ddl or d4T) for at least three months.
Each subject had a BMI below 19.8 kg • m-2, or had
experienced a wt loss > 10%. Those subjects who previously (within
30 d) had an opportunistic infection or diarrhoea, malignancy (within
3 y), active Kaposi sarcoma (within 3 mon), or therapy with anabolic
or catabolic agents (within 1 mon) were excluded. The final study
group consisted of 39 HIV-positive 25-50 y-old males.
Anthropometry
and dietary intake
Body wt was measured to ± 0.2 kg, ht to ± 0.5 cm, and skinfold thicknesses
to ± 2 mm. The skinfold measurement sites were the biceps (BSF), triceps
(TSF), subscapular and suprailiac. The percentage of fat (%FATDW)
was calculated using the age-adjusted equations reported by Durnin
and Womersley4, based on the log of the sum of these four
skinfolds. In addition, the measurement of the mid-arm muscle circumference
(MAMC) was defined as: MAMC (cm) = MAC (cm) - 0.314 x TSF (mm) where
MAC is the mid-arm circumference.
The subjects were also instructed by a registered
dietitian on completing a 4-d food diary. Analysis of nutritional
intake, kJ/kg and g protein/kg, were calculated using Nutritionist
III5.
Body
composition measurements
Total body potassium (TBK) was measured in vivo in
a multi-detector whole body counter as described previously6.
The measurement is based on the natural radioactive fraction (40K,
0.018%) of K. Because more than 97% of body K is normally intracellular,
the TBK measurement provides an index of body cell mass (BCM). The
precision of this method is 0.7% for adult-sized phantoms and <1.2%
for humans. In this study, TBK is expressed in absolute grams and
as a percentage of ‘normal’ when adjusted for age, sex and
body size7.
The total body fat (FAT), %FAT, non-bone lean tissue
mass (LEAN) and bone mineral content (BMC) were measured by dual-energy
X-ray absorptiometry (DXA), using a Hologic QDR-2000 scanner operated
in the single beam mode and whole body software version 5.57. The
reported precisions for body composition analysis are of the order
of I -I .5%; the whole body dose is <0.01 mSv8.
Results
The mean BMI was 20.8 ± 2.4 kg • m-2 ranging from
16.6 to 26.1. The CD4+/mm3 counts ranged from 2 to 531,
with a mean of 126; 30 subjects had counts below 200 (Table 1). The
average %FATDXA was 14.3% of body wt. Body K averaged 130.0
g (range 96.4 to 196.1 g). When normalized for body size, sex and
age, TBK averaged 90.2% ± 10.8% of the expected normal range.
The daily dietary intake, normalized for body wt, ranged from 105-337
kJ/kg, with an average of 199 ± 52 kJ/kg. There was also a three-fold
range in the estimated daily protein intake, 0.9 to 1000 3.0 g/kg,
averaging 1.8 ± 0.6 g/kg.
Table 1. Descriptive statistics for the anthropometric,
CD4, skinfold and body composition measurements.
| Anthropometry: |
| |
Age (y)
|
wt (kg)
|
ht (cm)
|
BMI
|
CD4+
|
|
| Mean |
35.1
|
65.0
|
176.5
|
20.85
|
126
|
|
| SD |
6.5
|
8.4
|
6.0
|
2.40
|
144
|
|
| Min |
25.2
|
51.5
|
164.5
|
16.55
|
2
|
|
| Max |
47.4
|
87.6
|
194.5
|
26.06
|
531
|
|
| Skinfold measurements: |
| |
BSFmm
|
TSFmm
|
MACcm
|
SSSmm
|
SISmm
|
%FAT
|
| Mean |
4.0
|
7.2
|
28.2
|
11.4
|
8.3
|
15.7
|
| SD |
1.3
|
2.8
|
2.9
|
3.3
|
6.3
|
4.1
|
| Min |
2.5
|
3.0
|
22.5
|
5.5
|
3.0
|
8.8
|
| Max |
8.0
|
15.0
|
34.5
|
18.0
|
27.0
|
26.6
|
| Body composition
measurements: |
1000
| |
TBK (g)
|
%TBK
|
Lean (kg)
|
BMC (g)
|
Fat (g)
|
%FAT
|
| Mean |
130.0
|
90.2
|
52.2
|
2427
|
9554
|
14.3
|
| SD |
18.7
|
10.8
|
5.9
|
316
|
4267
|
5.3
|
| Min |
96.4
|
66.5
|
42.5
|
1644
|
2765
|
5.4
|
| Max |
196.1
|
113.3
|
74.0
|
3240
|
19696
|
25.0
|
%TBK = % of normal TBK. %FAT = (fat/wt) x 100.
There were no correlations of LEAN or FAT with nutritional
intake, as monitored by the 4-d food diary. 1000 Furthermore, CD4+
counts were also not correlated with any of the body composition parameters
measured in this study. There were, however, significant correlations
between the anthropometric, skinfold, and body composition measurements.
The MAC and BMI were strongly correlated with each other, yet the
skinfold measurements were not generally correlated with any of the
direct measures of body composition obtained by the DXA or TBK measurements.
Height was related only with the BMC, whereas body wt was correlated
with TBK, MAC and BMI. Total body K was significantly correlated with
the LEAN and to a lesser degree with two anthropometric indices, BMI
and MAC. The LEAN was equally correlated with the BMC compartment
as was BMI and MAC. There was a weak association between FAT and the
TSF measurement, but not the other skinfolds.
The correlation between TBK and MAC was substantially
improved when the MAC value was adjusted for the arm fat content by
using the TSF value. The relationship between TBK and MAMC is shown
in Fig 1. The linear regression results are: TBK (g) = 5.35 x MAMC
(cm) - 8.2, r = 0.82, SEE = 10.9 g. For comparison, the relationship
observed between the two independent reference measures of lean tissue
mass is shown in Figure 2. The linear regression results are: TBK
(g) = 2.85 x LEANDXA - 18.7, r = 0.90, SEE = 8.2 g.
Figure 1. The relationship between total body
potassium (TBK) and the mid-arm muscle circumference (MAMC) in HlV-positive
males.
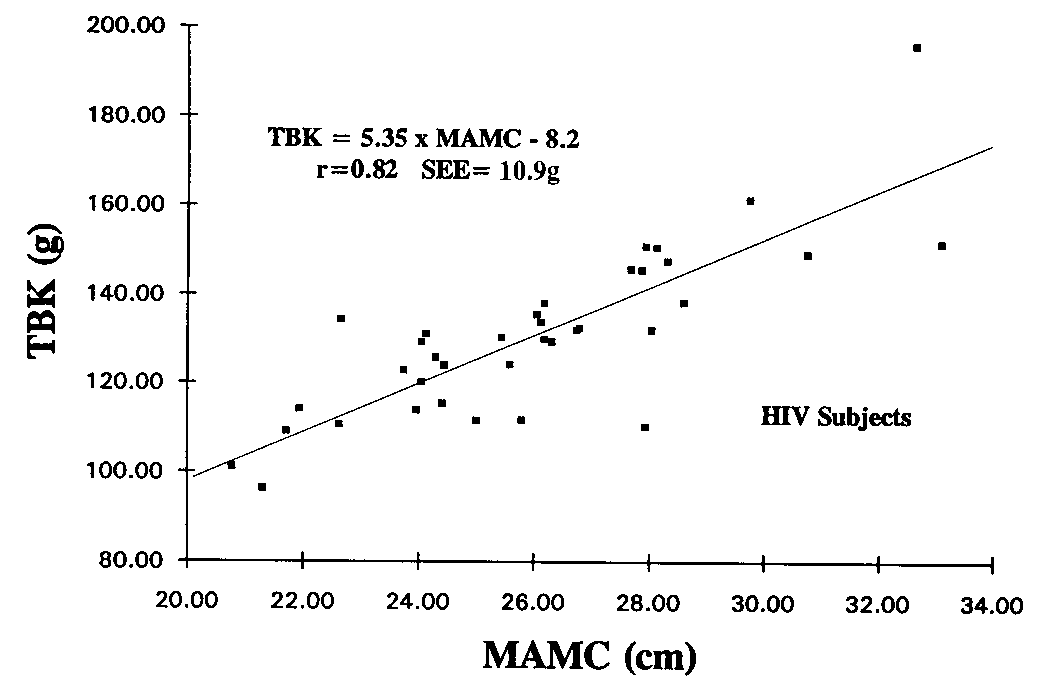
Figure 2. The relationship observed between
the lean compartment (LEAN) by dual-energy X-ray absorptiometry (DXA)
and body potassium (TBK) by 40K counting.

Various combinations of the skinfold, wt, and ht measurements
were examined for a relationship to %FAT. The TSF combined with BMI
provided the best anthropometric equation: %FATDXA = I
.106 x TSF (mm) + 0.85 x BMI (kg/cm2) 11.13,r=0.77,SEE=3.5%.
Discussion
Our findings show that male adults with HIV wasting
syndrome have significantly depleted BCM as judged by a loss of body
K. This finding is in agreement with those of other studies observed
for the AIDS population9-11. Additional evidence for a
depleted BCM is provided by the TBK/FFM (fat free mass) ratio, where
FFM was independently derived from body weight minus FATDXA.
In this study group, the TBK/FFM ratio varied from 2.10 to 2.73 g/kg,
averaging 2.37 ± 0.16 g/kg. The traditional ratio,
established by Forbes and Lewis12, for healthy young males
is 2.66 g/kg, about 12% higher than that observed in HIV males of
this study. A depletion of BCM, however, is not immediate in HIV infection,
since 18/39 subjects had TBK within the normal range. It is worth
noting, however, that all those subjects with a CD4+ count above 200
also had %TBK within the normal range. No pattern was evident for
%FAT vs CD4+ counts except that all the HIV subjects had a substantially
lower %FAT than is normally seen in a healthy male population of a
similar age. It was evident that this wt loss consisted of both depleted
body K stores and FAT. Therefore, a reliable measurement of body composition
in this group can provide a useful index to assist in nutritional
assessment, only if some measure of both LEAN and FAT are obtained.
Although the more robust techniques of whole body
counting and DXA can clearly provide precise measures of body composition,
these instruments are not always available to the HIV patient, especially
in many of the world's less developed countries. For the m 1000 easurement
of FAT, established anthropometric equations are usually considered
as only suitable for the study of normal healthy subjects. In the
malnourished patient, however, these relationships falter, most likely
due to changes in visceral fat that are not proportional to total
fat and are not detectable by anthropometry, ie skinfold measurements.
We have found, however, that reasonable estimates for the body K stores
and %FAT can be obtained using BMI, MAC and TSF measurements. In this
respect, a clinical anthropometric assessment of the HIV patient may
provide an appropriate alternate estimate of the muscle and fat masses
when more direct measurements are not available.
Acknowledgments—We wish to acknowledge J. Joo and J. Pratt for assistance with
the body composition measurements and S. Charboneau for editorial
review of the manuscript.
This work is supported, in part, by the Genentech,
Inc. and by the US Department of Agriculture, Agricultural Research
Service under Cooperative Agreement # 58-6250-l-003 with Baylor College
of Medicine. The contents of this publication do not necessarily reflect
the views or policies of the USDA or Genentech, nor does mention of
trade names, commercial products, or organizations imply endorsement.
Reference
- Nahlen AL, Chu SY, Nwanyanwu OC, Berkelman RL,
Martinez SA, Rullan IV. HIV wasting syndrome in the United States.
AIDS 1993; 7:183-188.
- Zangerle R, Reibnegger G, Wachter H, Fuchs D. Weight
loss in HIV-I infection is associated with immune activation. AIDS
1993; 7:175-181.
- Kotler DP. Malnutrition in HIV infection and AIDS.
AIDS 1993; 3:S175-S180.
- Durnin JVGA, Womersley J. Body fat assessed from
total body density and its estimation from skinfold thickness: measurements
on 481 men and women aged from 16 to 72 years. Br J Nutr 1974:32:77.
- Nutritionist III (version 7.0). N-Square Computing,
Inc., Salem, OR, USA, 1990.
- Ellis KJ, Shypailo RJ. Whole body potassium measurements
independent of body size. In: Ellis KJ, Eastman JD, eds. Human Body
Composition, New York: Plenum Press, 1993: 371 -375.
- Ellis KJ. Reference man and woman more fully characterized:
variations on the basis of body size, age, sex and race. Biol Trace
Element Res 1990; 26:385 400.
- Ellis KJ, Shypailo RJ, Pratt JA, Mersmann H, Pond
WG. Accuracy of DXA-based body composition measurements in pediatric
studies. In: Human Body composition: Methods, Models and Assessment.
New York: Plenum Press, 1993: 153-156.
- Kotler DP, Tiemey AR, Wang J, Pierson RN Jr. Magnitude
of body-cell-mass depletion and the timing of death from wasting
in AIDS. Am J Clin Nutr 1989; 50:444-447.
- Kotler DP, Tierney AR, Brenner SK, Couture S, Wang
J, Pierson RN Jr. Preservation of short-term energy balance in clinically
stable patients with AIDS. Am J Clin Nutr. 1990; 51:7-13.
- Kotler DP, Wang J, Pierson RN Jr. Body composition
studies in patients with the acquired immunodeficiency syndrome.
Am I Clin Nutr. 1985; 42:1255-1265.
- Forbes GB, Lewis AM. Total sodium, potassium and
chloride in adult man. J Clin Invest 1956; 6:596-600.

Copyright © 1995 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
 to the top
to the top
0