Asia Pacific J Clin Nutr (1993) 2, Suppl 1, 15-20
Natural antioxidants and atherosclerosis
Roland
Stocker PhD
Heart Research Institute, Missenden
Road, Camperdown, NSW 2050, Australia.
The precursors of fibrous atherosclerotic plaques
are fatty streaks, characterized by accumulation of fat laden macrophages
beneath an intact endothelium. These macrophages are derived from
monocytes in the circulating blood and the lipid is derived from
plasma low density lipoprotein (LDL). But LDL is poorly taken up
by monocytes/macrophages in vitro unless it has been oxidatively
modified. Hence the phypothesis has developed that one determinant
of atherosderosis is whether LDL becomes oxidized by free radicals
in the subendothelial space. An epidemiological study of 12 European
sub-populations which all have about the same plasma cholesterol
concentration but quite different incidences of coronary heart disease
(CHD) showed a significant inverse correlation of plasma a -tocopherol with CHD. In several
animal models, vitamin E or some other antioxidants attenuate experimental
atherosclerosis. Each particle of LDL contains about 8-12 molecules
of tocopherol, 0.5 to 1 molecule of ubiquinol-10 and small amounts
of carotenoids but other antioxidants in the extracellular fluid,
notably (water-soluble) ascorbate protect against oxidative damage
in in vitro experiments with human blood plasma. The ascorbate presumably
acts by regeneration a -tocopherol from its one-electron
oxidation product, the a-tocopheroxyl radical. The author
found that the small amounts of ubiquinol present in LDL offer important
protection against oxidation. Unlike vitamins C and E, ubiquinol
is biosynthesized by humans but it is also obtained from the diet
(some fatty fish are the richest sources). The ubiquinol content
of plasma LDL was increased 4-fold by giving volunteers ubiquinone.
Their plasma LDL was subsequently found more resistant against oxidation.
Background
Atherosclerosis represents a major form of ischaemic
heart disease (IHD), the leading cause of death in western countries.
Elevated levels of low density lipoprotein (LDL) cholesterol are an
important risk factor for atherosclerosis. This can be concluded from
clinical observations on patients with familial hypercholesterolemia,
a single, well-defined gene defect involving the LDL receptor. The
premature atherosclerosis observed in these patients must be, directly
or indirectly, related to their elevated levels of LDL in plasma.
While undoubtedly important, hypercholesterolemia is however clearly
not the single causative factor for atherosclerosis. Other variables
contribute (and probably interact) with hypercholesterolemia in determining
the overall risk for atherosclerosis.
Among the additional risk factors, lifestyle and nutrition
are of interest. Epidemiological evidence suggests that the age-adjusted
IHD mortality rate can vary substantially in populations of different
countries. For example, northern Europeans have a much higher rate
of IHD than people in Italy, Spain or France. It has been suggested
that antioxidants present in the fruit and vegetables-rich Mediterranean-type
diet provides some protection against heart disease. Indeed, supporting
evidence for this comes from the WHO Monica project in which the plasma
concentrations of cholesterol and antioxidants in European sub-populations
were measured and correlated to their risk of IHD incidents. Among
12 sub-populations with similar cholesterol levels, the plasma concentrations
of atocopherol (TOH) strongly and inversely correlated with the mortality
rate1. A negative correlation was also observed with plasma
vitamin C; however, it was weaker than that observed for TOH and disappeared
when smoking was introduced as an additional, separate risk factor1.
Plasma levels of vitamin A and b -carotene did not correlate with
the mortality rate. Additional evidence for a beneficial effect of
dietary antioxidants (vitamins C and E and b -carotene) on cardiovascular disease
in humans has been reviewed recently2.
Animal studies further support the idea that antioxidant
nutrients have a beneficial effect on atherosclerosis. Some 40 years
ago, vitamin C-deficient guinea pigs were used as an animal model
of atherosclerosis3. It was reported that in these deficient
animals the ground substance of the sub-endothelial space was disturbed
and lipid deposited in the sub-endothelial space3. The
latter could be reversed by providing the animals with vitamin C in
the diet4. The same group of scientists also reported that
human post-mortem lesion material was deficient in vitamin C5,6.
Other groups using guinea pigs as an animal model for atherosclerosis
failed to confirm that simple vitamin C deficiency was associated
with lipid deposition in the sub-endothelial space7,8.
It was observed however, that a combination of vitamin C deficiency
and a high fat diet caused lipid deposition in the sub-endothelial
space of guinea pigs that were attenuated by vitamin C supplementation8.
Several groups have shown that in different rabbit models of atherosclerosis,
administration of vitamin E attenuates the progression of the disease
(eg9-11). Treatment with synthetic antioxidants such as
probucol12,13 or butylated hydroxytoluene14
also inhibits the development and progression of atherosclerosis in
various animal models. Interestingly however, in all these cases (except
that with butylated hydroxytoluene) antioxidant administration also
had a significant hypolipaemic activity.
Low density
lipoprotein oxidation
How can the beneficial role indicated above for antioxidants
in atherosclerosis be rationalized? It is now widely accepted that
the precursors of fibrous plaques and more complicated atherosclerotic
lesions are the fatty streaks, ie the accumulation of fat-laden monocytes/macrophages
(referred to as foam cells) beneath an intact endothelial cell layer
(reviewed in 15). These phagocytes are derived from the
circulation, following adherence to and penetration of the arterial
endothelium. Like other circulating macromolecules, LDL transverses
endothelial cells and accumulates in the subendothelial space. However,
before LDL is taken up by monocytes/macrophages at sufficiently high
rates to cause foam cell formation, the lipoprotein must undergo some
form of 'modification'. While a number of chemical 'modifications'
have been shown to have this effect, there is mounting evidence that
'oxidative modification' is biologically important15. The
presence of highly effective antioxidant defences in human blood (see
below) has led to the 'assumption' that oxidative modification of
LDL associated with atherogenesis takes place in the subendothelial
space15, even though there is surprisingly little direct
evidence for this. The beneficial effect of antioxidants on atherosclerosis
is most often attributed to their protective action on LDL oxidation2,15.
While not discussed here, other explanations can not be excluded (see
eg16); also, oxidative LDL modification resulting in an
atherogenic lipoprotein may be achieved by non-radical oxidants such
as hypochlorite, against which antioxidants offer little protection17.
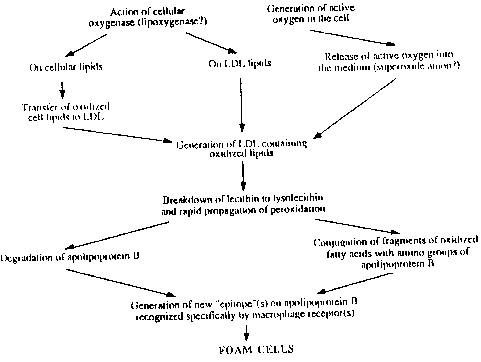
The precise mechanism by which LDL oxidation proceeds
in the subendothelial space is still unclear, and a number of free
radical-mediated mechanisms have been proposed15 (Figure
1). These include the generation of reactive oxygen species (ROS)
by cells of the subendothelial space causing (per)oxidation of LDL
lipids, the possible involvement of transition metals for the catalysis
of highly reactive radicals derived from the primary ROS18,
and the action of cellular lipoxygenases on either LDL lipids directly19,
or on cellular lipids with subsequent transfer of oxidized lipids
to LDL15 (but see20). Following initiation,
LDL lipid peroxidation is thought to propagate, leading to massive
lipid oxidation, with breakdown of oxidized lipids to reactive moieties
that can modify amino acid residues on the protein in LDL, thereby
generating new epitope(s) recognized by specific macrophage receptors15.
Figure 1. Mechanisms thought to lead to oxidative
modification of LDL by cells (Steinberg et al. 1989).
Low density
lipoprotein antioxidation
Non-proteinaceous
antioxidants surrounding lipoproteins
As it is difficult to gain access to extracellular
material surrounding LDL in the sub-endothelial space, most studies
on antioxidant defences in extracellular fluids have used human blood
plasma as a model. A number of proteinaceous defences that are present
in plasma are designed primarily to eliminate transition metals from
participating in unwanted redox reactions leading to deleterious effects
(reviewed in 21,22). Extracellular fluids also contain
non-proteinaceous antioxidants21,22. Among them, ascorbate
(AH-, the reduced form of vitamin C) appears to be the most efficient
aqueous antioxidant.
In an attempt to assess qualitatively the relative
contribution of the various non-proteinaceous antioxidants in extracellular
fluids, we exposed freshly obtained human plasma to a constant chemical
source of aqueous radical oxidants and examined the temporal consumption
of antioxidants and formation of oxidatively damaged lipids23.
We observed that AH- was oxidized first, followed by protein-thiols,
bilirubin, uric acid and a -tocopherol (TOH, the most active form of vitamin E). Aqueous (peroxyl)
radical-induced peroxidation of lipoprotein lipids in plasma was detectable
only following complete consumption of AH-, despite the presence of
normal amounts of TOH and other antioxidants23. Considering
the generally accepted view of TOH's action24, it was surprising
to find formation of significant amounts of lipid hydroperoxides (LOOH,
a primary form of oxidized lipids) in the presence of vitamin E. Additional
work on the antioxidant properties of AH- suggests that this antioxidant
represents the first and most efficient line of antioxidant defence
in human plasma against some (but not all) aqueous, radical oxidants
(reviewed in 25). We have recently obtained similar efficient
antioxidation by AH- in rat thoracic lymph, another extracellular
fluid (D. Mohr, Y. Umeda, T. Redgrage, R. Stocker, unpublished). It
therefore seems reasonable to assume that AH- plays a similarly important
role as an aqueous antioxidant in the extracellular fluid of the sub-endothelial
space in humans.
Low
density lipoprotein-associated antioxidants
To understand the mechanism of how LDL lipids become
oxidized to generate an atherogenic lipoprotein, information on how
LDL itself is protected against oxidation is important. LDL contains
a surface coat consisting of a monolayer made up of phospholipids,
free cholesterol and a protein (referred to as apoprotein B100). This
surface coat surrounds a core containing the neutral cholesterylesters
and triglycerides. The composition of human LDL is given in Table
1. As can be seen, each LDL particle contains about 8-12 molecules
of TOH, 0.5-1 molecules of ubiquinol-10 (QH2) and small
amounts of carotenoids. Thus, TOH is by far the most abundant antioxidant
associated with human LDL.
Table 1. Composition of human LDL.
| Components |
|
Relative mass (%) |
Molecule/particle |
| Protein |
Apo B-100 |
23.4 |
1 |
| Lipids |
Phospholipids |
20.6 |
800 |
| |
Cholesterol |
9.0 |
500 |
| |
Cholesterylesters |
41.7 |
1500 |
| |
Triglycerides |
5.3 |
180 |
| Antioxidants |
a -Tocopherol |
|
8-12 |
| |
Ubiquinol-10 |
|
0.5-1.0 |
| |
Lycopene |
|
0.7 |
| |
b -Carotene |
|
0.4 |
Because TOH is the major lipid-soluble antioxidant
in LDL, it is generally also regarded as the most important lipid-soluble
antioxidant in this lipoprotein26 as well as in human plasma27.
In direct contradiction to this however, various groups have reported
that the amount of TOH in LDL does not correlate well with the lipoproteins
resistance against oxidation28-30.
Investigating in detail the early stages of LDL lipid
oxidation, we started to unravel the above-mentioned anomalies. We
discovered that the small amounts of QH2 present in LDL
offer outstanding protection against oxidation31. Thus,
exposing freshly isolated LDL to a constant flux of aqueous radicals,
LDL lipid peroxidation is inhibited substantially in the presence
of QH2, which is the first of LDL's antioxidants to be
consumed. Following consumption of QH2, lipid peroxidation
proceeds at high rates and in a radical chain process: despite the
presence of normal amounts of TOH, up to 20-40 molecules of oxidized
lipid can be formed for each oxidant 'hitting' an LDL particle31.
Peroxidation of LDL lipids via a chain reaction process in the presence
of TOH has also been observed by Niki and co-workers32,
in line with our previous observations in human blood plasma23.
Adding AH- to the oxidizing LDL completely prevents lipid oxidation,
demonstrating that this vitamin is indeed a very efficient antioxidant31.
The observed complete prevention of LDL lipid peroxidation by AH-
is most likely due to the ability of this antioxidant to regenerate
TOH from its one-electron oxidation product, a -tocopheroxyl radical (TO) (see below);
AH- is unable to regenerate QH233.
Unlike the vitamins C and E, QH2 can be
synthesized by humans, and part of the QH2 in our body
is derived from such biosynthesis, the other from dietary sources.
Certain species of fish (eg sardines and mackerels) are the richest
sources of QH2. We therefore tested whether LDL's content
of QH2 can be increased by dietary supplementation and
whether such QH2-supplemented LDL is more resistant towards
oxidation. As QH2 in foods is present primarily in its
(two electron) oxidized form ubiquinone-10 (Q, which itself is not
an antioxidant), we administered volunteers with 3 x 100 mg Q per
day. Such supplementation resulted in an approximately 4-fold increase
in the levels of QH2 (the reduced form of Q that is antioxidant-active),
but did not alter the content of other antioxidants in LDL34.
More importantly, QH2-supplemented LDL was significantly
more resistant against oxidation compared to the nonsupplemented LDL34.
These results strongly support the efficacy and importance of QH2
in LDL antioxidation.
Dietary supplementation with TOH also results in an
increase in its concentration in LDL, although it appears that high
doses have to be used to achieve a similar relative increase compared
with Q supplementation30-34. We have made similar observations
with a -tocotrienol, another form of vitamin E
that is present in certain foodstuff and is as antioxidant-active
as TOH35. As alluded to earlier however, the relationship
between TOH supplementation and LDL oxidizability is weak, in contrast
to the situation with QH2. Other groups have studied the
protective effect of antioxidant nutrients on in vitro LDL oxidizability.
Table 2 shows that in most cases the oxidizability of LDL is inhibited
completely or reduced in the presence of supplemented antioxidants
and using different types of oxidizing conditions. A word of caution
appears appropriate here regarding potential problems with in vitro
studies using lipid-soluble antioxidants. Due to their physical properties,
'physiologically correct' incorporation of these lipophilic substances
when added in vitro (eg in ethanolic solution) is not guaranteed.
We have observed significantly different behaviour of in vitro versus
in vivo QH2-enriched LDL34, and this may explain
why one group observed a protective activity of relatively large amounts
of added b -carotene on LDL oxidation in vitro36.
In our hands, b-carotene is not an efficient
in vitro lipid antioxidant. In any case, it is commendable to substantiate
in vitro findings with appropriate in vivo supplementation studies.
Table 2. Antioxidant nutrients and LDL's oxidizability.
| Nutrient |
Mode |
Supplementation |
Fold
Increase |
Oxidizing
conditions |
Oxidizability |
Reference |
| |
|
Dose
(mg/d) |
Duration
(days) |
|
|
|
|
| Vitamin C |
In Vivo |
- |
- |
- |
ROO· /PMN |
Inhibited |
Stocker et al. 1991 |
| |
In Vivo |
l500 |
28 |
2 |
Smoking |
Reduced |
Harats et al. 1990 |
| Vitamin E |
In Vitro |
- |
- |
3 |
Cu2+ |
Reduced |
Esterbauer et al. 1991 |
| |
In Vivo |
1450 |
3 |
2.5 |
Maurine MŲ |
Reduced |
Jessup et al. 1990 |
| |
In Vivo |
600 |
28 |
n.d. |
Smoking |
Reduced |
Harats et al. 1990 |
| |
In Vivo |
100-800 |
21 |
3 |
Cu2+ |
Reduced |
Esterbauer et al. 1991 |
| |
In Vivo |
667 |
7 |
2.4 |
Cu2+ |
Reduced |
Princen et al.1992 |
| b -Carotene |
In Vitro |
- |
- |
13 |
Cu2+ |
Reduced |
Jialal et al. 1991 |
| |
In Vivo |
40 |
14 |
16 |
Cu2+ |
Not altered |
Princen et al. 1992 |
| CoQ1OH2 |
In Vivo |
300 |
10 |
4 |
ROO* |
Reduced |
Mohr et al. 1992 |
Pro-oxidant
activity of a-tocopherol
Examining the precise role of TOH in LDL lipid antioxidation
in the absence of AH- and following QH2 consumption, we
observed that under conditions where LDL was exposed to a constant
flux of aqueous or lipophilic radicals, the rate of lipid hydroperoxide
formation actually decreased as TOH was consumed37.
Furthermore, LDL supplemented with TOH peroxidized at a higher rate
than the corresponding control, non-supplemented lipoprotein37.
In fact, the rate at which cholesteryllinoleate, the major single
substrate for oxidation in LDL (Table 1), becomes oxidized is directly
proportional to the lipoprotein's content of TOH38. These
findings clearly demonstrate that TOH alone is not an efficient
antioxidant for LDL; rather it can act as a potent pro-oxidant in
the isolated lipoprotein.
These findings led to a detailed theoretical and experimental
analysis of the molecular action of TOH in LDL. To our surprise, the
results obtained with isolated LDL do not support the conventional
mode of action of TOH, whereby this antioxidant scavenges a lipid
radical produced when a peroxidation-initiating (aqueous) radical
reacts with lipids, and where the resulting TO· is eliminated by reaction with another lipid or initiating radical.
In this conventional mode of action, at least one molecule of LOOH
is formed for each molecule of TOH consumed. In contrast, we observed
that TOH in fact acts as both a phase transfer (ie it captures
and thereby transfers aqueous radicals into the lipid phase) and chain
transfer agent (ie it produces the lipid peroxidation chain-carrying
radical) during radical-mediated LDL oxidation38. We have
termed this novel mode of TOH's action as tocopherol-mediated peroxidation
(TMP). TMP, rationalized and explained explicitly in 38
and its role in LDL antioxidation is illustrated in Figure 2.
Figure 2. Anti- and pro-oxidation of LDL lipids
by a -tocopherol.
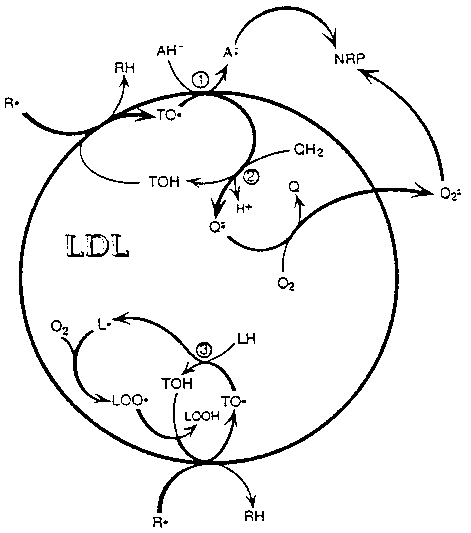
As the most reactive component, TOH becomes oxidized
(to TO· ) when LDL encounters an oxidation initiating radical (R·
). The TO·
formed can undergo three reaction pathways, all of which result in
regeneration of TOH. Pathways 1 and 2 represent antioxidation,
whereas pathway 3 represents pro-oxidation of TOH. In the antioxidation
pathways, TO· reacts with either aqueous AH- or QH2. The ascorbyl- (A· ) or semiquinone- (Q· ) radicals formed decay (directly or indirectly) giving rise to non-radical
products (NRP) in the aqueous phase while eliminating the harmful
R· . In the absence of AH- and QH2 the pro-oxidant pathway
is followed, where the long-lived TO· is forced to react with LH producing
a carbon-centered lipid radical (L· ). Under aerobic conditions, L· will add to O2 producing LOO· that propagates lipid peroxidation
by reacting with another TOH (shown) or LH (not shown) to form (LOOH).
Nutritional
implication of tocopherol-mediated peroxidation
From our model (Figure 2)38 it follows
that in most, if not all mammalian tissues and fluids, (where AH-
and QH2 are ubiquitous) TOH in fact is a better
antioxidant than previously assumed; LOOH are not formed during normal
action of TOH (pathways 1 and 2). It is clear however, that for such
efficient LDL antioxidation, TOH requires 'partner' molecules that
either reduce (eliminate) TO· and/or eliminate the radical character from
the isolated LDL particle37,38. In light of this and the
fact that LDL from healthy people is already rich in TOH, I consider
dietary attempts aimed exclusively at further increasing the lipoprotein's
concentration of TOH unlikely to be most efficient. Since QH2
and AH- are the only natural compounds known at present to eliminate
TO· and
therefore TMP, a more promising strategy seems to be supplementation
with these two antioxidants, in combination with TOH. There
may be other compounds in extracellular fluids (eg the reduced form
of vitamin K1, albumin-bound bilirubin) that can also reduce
and/or eliminate TO· in LDL, and the concentration of
some of which may be increased by appropriate dietary means. I consider
supplementation with Q as a particularly attractive and exciting possibility
because the 'normal' circulating levels of this highly efficient antioxidant
are low (see Table 1), and its concentration can easily be elevated
to more than one molecule per LDL particle34. Achieving
the latter would not only guarantee efficient elimination of TO· , but also enhance the possibility of additional LDL antioxidation through
regeneration of QH239
Acknowledgments--The work and contribution of the colleagues and co-workers from the author's
laboratory is gratefully acknowledged. The work was supported in part
by the Australian National Health & Medical Research Council grant
910284 to R.S.
References
- Gey KF, Puska P, Jordan P, Moser UK. Inverse correlation
between plasma vitamin E and mortality from ischemic heart disease
in cross-cultural epidemiology. Am J Clin Nutr 1991; 53:326S-334S.
- Gaziano JM, Hennekens CH. Vitamin antioxidants
and cardiovascular disease. Current Opinion in Lipidology 1992;
3:291-294.
- Willis GC. An experimental study of the intimal
ground substance in atherosclerosis. Canad MAJ 1953; 69:1722.
- Willis GS. The reversibility of atherosclerosis.
Canad MAJ 1957; 77:106-109.
- Willis GC, Light AW, Gow WS. Serial arteriography
in atherosclerosis. Canad MAJ 1954; 71:562-568.
- Willis GC, Fishman S. Ascorbic acid content of
human arterial tissue. Canad MAJ 1955; 72:500-503.
- Gore I, Tanaka Y, Fujinami T, Goodman ML. Aortic
acid mucopolysaccharides and collagen in scorbutic guinea pigs.
J Nutr 1965; 87:311-316.
- Ginter E, Babala J, Cerven J. The effect of chronic
hypovitaminosis C on the metabolism of cholesterol and atherogenesis
in guinea pigs. J Atheroscler Res 1969; 10:341-352.
- Wilson RB, Middleton CC, Sun GY. Vitamin E, antioxidants
and lipid peroxidation in experimental atherosclerosis of rabbits.
J Nutr 1978; 108:1858-1867.
- Wojcicki J, Rozewicka L, Barcew-Wiszniewska B,
Samochowiec L, Juzwiak S, Kadlubowska D, Tustanowski S, Juzyszyn
Z. Effect of selenium and vitamin E on the development of experimental
atherosclerosis in rabbits. Atherosclerosis 1991; 87:9-16.
- Williams RJ, Motteram JM, Sharp CH, Gallagher PJ.
Dietary vitamin E and the attenuation of early lesion development
in modified Watanabe rabbits. Atherosclerosis. 1992; 92:153-159.
- Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima
A, Yoshida H, Kawai C. Probucol prevents the progression of atherosclerosis
in Watanabe heritable hyperlipidemic rabbit, an animal model for
familial hypercholesterolemia. Proc Natl Acad Sci USA 1987; 84:5928-5931.
- Carew TE, Schwenke DC, Steinberg D. Antiatherogenic
effect of probucol unrelated to its hypocholesterolemic effect:
evidence that antioxidants in vivo can selectively inhibit low density
lipoprotein degradation in macrophage-rich fatty streaks and slow
the progression of atherosclerosis in the Watanabe heritable hyperlipidemic
rabbit. Proc Natl Acad Sci USA 1987; 84:7725-7729.
- Bjrkhem I, Henriksson-Freyschuss A, Breuer O, Diczfalusy
U, Berglund L, Henriksson P. The antioxidant butylated hydroxytoluene
protects against atherosclerosis. ArteriosclerThromb 1991; 11:15-22.
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC,
Witztum JL. Beyond cholesterol: modifications of low density lipoprotein
that increase its atherogenicity. N Engl J Med 1989; 320:915-924.
- Boscoboinik D, Szewczyk A, Azzi A. a -Tocopherol (vitamin E)
regulates vascular smooth muscle cell proliferation and protein
kinase C activity. Arch Biochem Biophys 1991; 286:264-269.
- Hazell LJ, Stocker R. Oxidation of low-density
lipoprotein with hypochlorite causes transformation of the lipoprotein
into a high-uptake form for macrophages. Biochem J 1993; 290:165-172.
- Halliwell B, Gutteridge JMC. Free radicals in biology
and medicine. Oxford: Clarendon Press 1989:1-543.
- Belkner J, Wiesner R, Kuhn H, Lankin VZ. The oxygenation
of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett
1991; 279:110-114.
- Jessup W, Darley-Usmar V, O'Leary V, Bedwell S.
5-Lipoxygenase is not essential in macrophage-mediated oxidation
of low-density lipoprotein. Biochem J 1991; 278:163-169.
- Halliwell B, Gutteridge JMC. The antioxidants of
human extracellular fluids. Arch Biochem Biophys 1990; 280:1
- Stocker R, Frei B. Endogenous antioxidant defenses
inhuman blood plasma. In: Sies H, ed. Oxidative stress: Oxidants
and antioxidants. London: Academic Press, 1991 :213-243.
- Frei B, Stocker R, Ames BN. Antioxidant defenses
and lipid peroxidation in human blood plasma. Proc Natl Acad Sci
USA 1988; 85:9748-9752.
- Burton GW, Ingold KU. Vitamin E: application of
the principles of physical organic chemistry to the exploration
of its structure and function. Acc Chem Res 1986; 19:194-201.
- Frei B. Ascorbic acid protects lipids in human
plasma and low-density lipoprotein against oxidative damage. Am
J Clin Nutr 1991; 54:1113S-1118S.
- Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg
G. Role of vitamin E in preventing the oxidation of low density
lipoprotein. Am J Clin Nutr 1991; 53:314S-321S.
- Burton GW, Joyce A, Ingold KU. Is vitamin E the
only lipid-soluble, chain-breaking antioxidant in human blood plasma
and erythrocyte membranes? Arch Biochem Biophys 1983; 221:281-290.
- Jessup W, Rankin SM, De Whalley CV, Hoult JRS,
Scott J, Leake DS. a -Tocopherol consumption during low
density-lipoprotein oxidation. Biochem J 1990; 265:399405.
- Babiy AV, Gebicki JM, Sullivan DR. Vitamin E content
and low density lipoprotein oxidizability induced by free radicals.
Atherosclerosis 1990; 81:175-182.
- Dieber-Rotheneder M, Puhl H, Waeg G, Striegl G,
Esterbauer H. Effect of oral supplementation with D-a -tocopherol on the vitamin E content of human low density lipoproteins
and resistance to oxidation. J Lipid Res 1991; 32:1325-1332.
- Stocker R, Bowry VW, Frei B. Ubiquinol-10 protects
human low density lipoprotein more efficiently against lipid peroxidation
than does a -tocopherol. Proc Natl
Acad Sci USA 1991; 88:1646-1650.
- Sato K, Niki E, Shimasaki H. Free radical-mediated
chain oxidation of low density lipoprotein and its synergistic inhibition
by vitamin E and vitamin C. Arch Biochem Biophys 1990; 279:402-405.
- Frei B, Kim M, Ames BN. Ubiquinol-10 is an effective
lipid-soluble antioxidant at physiological concentrations. Proc
Natl Acad Sci USA 1990; 87:4879-4883.
- Mohr D, Bowry VW, Stocker R. Dietary supplementation
with coenzyme Q10 results in increased levels of ubiquinol-10 within
circulating lipoproteins and increased resistance of human low density
lipoprotein to the initiation of lipid peroxidation. Biochim Biophys
Acta 1992; 1126:247-254.
- Suarna C, Hood RL, Dean RT, Stocker R. Comparative
antioxidant activity of tocotrienols and other natural lipid-soluble
antioxidants in a homogeneous system, and in rat and human lipoproteins.
Biochim Biophys Acta 1993; 1166: 163-170.
- Jialal I, Norkus EP, Cristol L, Grundy SM. b -Carotene inhibits the oxidative modification of low-density
lipoprotein. Biochim Biophys Acta 1991; 1086:134-138.
- Bowry VW, Ingold KU, Stocker R. Vitamin E in human
low-density lipoprotein. When and how this antioxidant becomes a
pro-oxidant. Biochem J 1992; 288:341-344.
- Bowry VW, Stocker R, Tocopherol-mediated peroxidation.
The pro-oxidant effect of vitamin E on the radical initiated oxidation
of human low-density lipoprotein. J Am Chem Soc 1993; 115:6029-6044.
- Stocker R, Suarna C. Extracellular reduction by
41 ubiquinone-1 and -10 by human Hep G2 and blood cells. Biochim
Biophys Acta 1993; 1158:15-22.
- Harats D, Ben-Naim M, Dabach Y, Hollander G, Havivi
E, Stein O, Stein Y. Effect of vitamin C and E supplementation on
susceptibility of plasma lipoproteins to peroxidation induced by
acute smoking. Atherosclerosis 1990; 85:47-54.
- Princen HM, van Poppel G, Vogelezang C, Buytenhek
R, Kok FJ. Supplementation with vitamin E but not b carotene in vivo protects low density lipoprotein from lipid
peroxidation in vitro. Effect of cigarette smoking. Arterioscl Thromb
1992; 12:554-562.

Copyright © 1993 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
to the top