|
|
Asia Pacific J Clin Nutr (1993) 2, Suppl 1, 33-36

Regional differences in coronary
heart disease in Britain: do antioxidant nutrients provide the key?
A.J. Brown
PhD
Cardiovascular Research Unit, University
of Edinburgh, Hugh Robson Building, George Square, EH8 9XF, Edinburgh,
Scotland, UK
In Britain, there are large regional differences
in mortality rates from coronary heart disease which can not be
explained by established risk factors such as elevated levels of
blood cholesterol or high blood pressure. These regional differences
can to a large extent be explained by a cluster of inter-related
factors: a poor diet lacking in fresh fruit and vegetables, cigarette
smoking, and low socio-economic status. All of these factors are
associated with a low dietary intake and hence a low blood concentration
of antioxidant nutrients. Increased oxidative stress resulting from
a low antioxidant status may therefore be the common mechanism by
which these factors operate.
Introduction
Food technologists have long known about the involvement
of free radicals in rancidity and 'off' flavours in food. More recently,
there has been growing awareness among medical researchers of the
importance of free radicals in normal human metabolism and disease
processes. The mechanisms by which our immune system defends us against
bacterial infection is mediated by free radicals as is the way our
liver breaks down toxins. However, if production of free radicals,
whether from normal 'house-keeping' systems or from environmental
sources (eg smoking, radiation, dietary toxins) becomes so great that
the body's antioxidant defence system can no longer cope, a state
of 'oxidative stress' is said to exist. Oxidative stress has been
implicated in more than 50 human diseases including coronary heart
disease (CHD), cancer, diabetes, and rheumatoid arthritis1.
The fact that the diet provides crucial ingredients of the body's
antioxidant defence system (eg vitamins C and E, the carotenoids,
and selenium) is leading to a renaissance of research into these micronutrients.
In Britain, the Ministry of Agriculture, Fisheries
and Food (MAFF) have recognized that more information is needed about
the biological significance of these nutrients and their optimal levels
in the diet. The MAFF, as part of a Special Emphasis Programme, is
funding a number of projects which are investigating diverse aspects
of the antioxidant nutrients, many focusing on their relevance to
CHD. It will be some years yet before the fruits of this research
can be realized and published. This paper offers some background on
why dietary antioxidant nutrients and oxidative stress might explain
the high incidence of CHD in Britain.
Coronary
heart disease in Britain
As in many industrialized countries, CHD is still
the major cause of death in Britain. Particularly devastating in middle
age, CHD accounts for more than a third of all deaths in men aged
45-64 and about a fifth of all deaths in women in the same age group2.
Unlike other industrialized countries such as Australia and the USA,
Britain has experienced a much slower and later decline in the coronary
epidemic3. Considerable regional differences occur within
Britain. Indeed, Scotland and Northern Ireland have among the highest
CHD mortality rates in the world, and exhibit a 25% higher rate than
England and Wales2. Even within Scotland, mortality rates
vary two- to three-fold4.
Why do such large regional variations occur? The answer
is not only pertinent to Britain but should bring us closer to understanding
the underlying causes of CHD. The Scottish Heart Health Study was
designed in the early 1980s to try to explain these regional differences4.
Data was collected from 10 359 men and women (aged 4~59) living in
22 Scottish districts. Established risk factors such as blood pressure
and blood cholesterol levels did not account for the differences in
CHD mortality5, although they may still be important within
a region6. We and other groups believe that the reason
revolves around the antioxidant nutrients.
Fresh
fruit and vegetables
If the classic Mediterranean diet with an emphasis
on fruit, salad, and olive oil' is the ideal we should be striving
towards, the antithesis is the traditional Scottish diet. Largely
devoid of fresh fruit and green vegetables, the stereotypical Scottish
diet is low in fibre, high in saturated fat and incorporates many
fried foods. Habit, expense, and lack of availability all contribute
to the traditional Scots' low intake of fresh fruit and vegetables
which are the best dietary sources of many of the antioxidant nutrients.
Data derived from the Scottish Heart Health Study5 show
that standardized mortality rates from CHD for a given district are
significantly correlated with the proportion of people who did not
eat fresh fruit in the previous week or those who did not usually
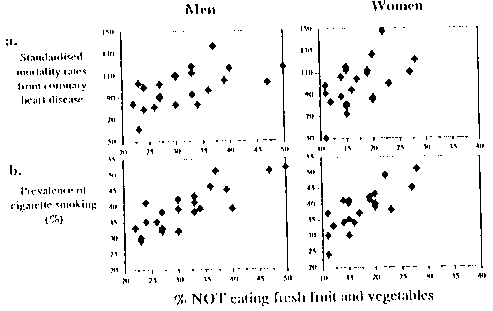
consume green vegetables (Figure 1a). Interestingly, lack of fresh
fruit and vegetable consumption appeared to have a more pronounced
influence on regional CHD mortality rates for women (slope=2.4) than
men (slope=1.2).
Figure 1. Relationship between the proportion
of people within a district who do not usually consume fresh fruit
and vegetables and:
- the district mean standardized mortality rate for
coronary heart disease. Equations of lines of best fit: y=1.24x+59.4
(men: r=0.56, p<0.01); y=2.39x+57.5 (women: r=;0.55; p<0.0).
- smoking prevalence in the district. Equations of
lines of best fit: y=0.738x + 15.9 (men: r=0.83,p<0.001); y=l.Olgx+20.7
(women r=0.78; p<0.001). Data for 22 districts were derived from
the Scottish Heart Health Study5
The Scot's dietary lack of fresh fruit and vegetables
is reflected in their low plasma levels of antioxidant nutrients.
Compared to Italian men, beneficiaries of the Mediterranean diet,
Scottish men on average had a 30% lower plasma vitamin E to cholesterol
ratio8. Plasma vitamin E concentrations are generally expressed
in relation to circulating cholesterol because they are transported
in the same lipoprotein particles and are therefore strongly associated.
Plasma concentrations of the principal water-soluble antioxidant,
vitamin C, were also much lower in the Scottish men (mean = 18.2m mol/l) compared to the Italians
(mean=38.0 m mol/1)8. Indeed, a third of the Scottish men displayed plasma
vitamin C levels below 11 m mol/l indicating biochemical depletion and
15% were on the threshold of scurvy (<6 m
mol/1)9. Occasional cases of scurvy are still reported
in Britain. The elderly, particularly men who live alone and eat a
poor diet, are the most commonly affected10. Even in 'developed'
countries, vitamin deficiencies have not yet been eradicated.
Dietary
fat composition
Further data from the Scottish Heart Health Study
on 20 of the 22 districts suggest that dietary fatty acid composition
may also be important in explaining the regional variation in CHD
mortality rates11. Regional CHD mortality rates were inversely
correlated with the proportions of linoleic acid in adipose tissue
(men r= -0.62, p<0.01; women r=-0.64, p<0.01). Co-ordinates
from two districts, one with the highest CHD mortality rate and the
other with the lowest, gave considerable weight to the correlation
and when these were removed from the analysis, the significant association
disappeared (men: r=-0.25, NS; women: r=--0.26, NS). Previous reports
of the inverse relationship between adipose tissue linoleate and CHD
risk12,13 have been more convincing. Adipose tissue linoleate
reflects long-term dietary intake of linoleic acid14, the
principal polyunsaturated fatty acid in the diet. However, because
diets rich in polyunsaturated fatty acids also tend to contain large
amounts of vitamin E15, adipose tissue linoleate may merely
be a proxy measure of long term vitamin E intake.
Dietary
fibre
In the Scottish Heart Health Study, risk from CHD
was significantly lower at higher intakes of fibre, b -carotene, and vitamins A, C, and
E for men, but only lower for fibre in women16. CHD risk
in this study was based on subjects who had been identified by the
WHO Chest Pain Questionnaire and therefore were unlikely to have changed
their lifestyle (diet, smoking habits, physical activity) at the time
of interview as a result of medical advice. Diet was assessed by a
food frequency questionnaire which was designed to target fibre intake17,18
rather than consumption of antioxidant nutrients. Food intake methodology
is notoriously fraught with difficulties19 and dietary
fibre is not a homogenous entity20. Moreover, fibre is
thought to exert its protective influence on CHD principally by lowering
blood cholesterol levels. However, the reduced risk of CHD with higher
fibre intake observed in the Scottish Heart Health Study was independent
of serum cholesterol concentrations16. Fibre itself may
be an indicator of overall antioxidant nutrient intake, since the
main sources of these nutrients (fruit and vegetables) contributed
to about a half of total fibre intake21. Indeed, when the
antioxidant nutrients (b -carotene, vitamins A, C, and E) were considered together, a significant
improvement in CHD risk was seen in both men and women at higher intakes16.
Cigarette
smoking
Smoking, a classical risk factor, was strongly associated
with CHD mortality rates in the Scottish Heart Health Study (men:
r=0.66, p<0.001; women: r=0.88, p<0.001). Cigarette smokers
have lower concentrations of antioxidant nutrients in their blood
when compared to non-smokers. Plasma carotenoids such as a - and b -carotene, and b -cryptoxanthin are all lower in smokers than non-smokers as is the plasma
vitamin E to cholesterol ratio22. Smokers also have a drastically
reduced level of plasma vitamin C21 and a higher ratio
of oxidized to reduced vitamin C (dehydroascorbate: ascorbate)23.
The lower levels of antioxidant nutrients seen in the blood of smokers
results from a combination of two factors. Firstly, smokers tend to
have a poorer diet than nonsmokers as evidenced by the highly significant
relationship between smoking prevalence and the lack of fresh fruit
and vegetables in the diet (Figure 1b). Secondly, smoking itself exerts
many deleterious effects: each puff on a cigarette has been estimated
to contain of the order of a million billion free radicals24.
Socio-economic
factors
Socio-economic factors also play a part in explaining
regional differences in CHD mortality. In Britain, death from a wide
range of diseases including CHD is unequally distributed between the
social classes25. In the Scottish Heart Health Study, social
factors like male unemployment and low social class explained most
(73%) of the regional variation in CHD mortality26. Other
studies have been fortunate if they could explain 50% of CHD mortality
using a battery of classical risk factors27. Although social
variables may tell us who is at greatest risk, they have limited value
in revealing the aetiology of the disease. For that, more biochemical
measures are required.
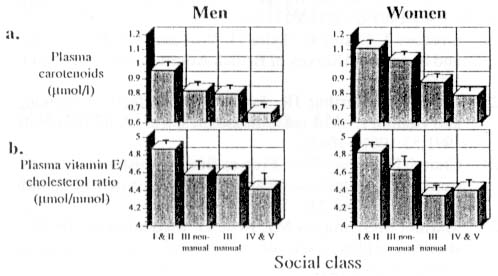
Results from the British Adult Survey22
show that there was a downwards gradation in blood concentrations
of antioxidant nutrients from professional (class I and II) to unskilled
manual workers (class IV and V) (Figure 2). Furthermore, the unemployed
had substantially lower concentrations of antioxidant nutrients in
their blood than workers. On average, unemployed 6 men had a 25% lower
concentration of plasma carotenoids and a 14% lower vitamin E to cholesterol
ratio. These differences again reflect a combination of lower intakes
of antioxidant nutrients22 and the destructive 7 effects
of smoking since smoking is more common among lower socio-economic
groups21,22.
Figure 2. Plasma concentrations of antioxidant
nutrients by social class. Social class is based on the occupation
of the head of household ranging from social class I (professional)
to social class V (unskilled manual). Each column represents data
(mean ± SEM) collected from at least 120 subjects. Data were derived from the
British Adult Survey22
- Plasma carotenoids comprising b -carotene, a -carotene, lycopene, and b -cryptoxanthin.
- Plasma vitamin E to cholesterol molar ratio.
The measure of social class based on the head of the
8 household's occupation has been the focus of much criticism in recent
years. Census data such as not having a car, overcrowding, and unemployment
are better indicators of deprivation than social class, and offer
a g promising basis for explaining health differences28.
Concluding
remarks
CHD is a multi-factorial disease which has kept researchers
guessing for more than a century. It is very doubtful that a single
factor will every fully explain CHD, but antioxidant nutrients may
come closer than the more established risk factors. Blood cholesterol
levels and blood pressure do not account for the regional differences
in CHD mortality observed in Britain. These regional differences can
to a large extent be explained by a cluster of inter-related factors:
poor diet, smoking, and low socio-economic status. Increased oxidative
stress resulting from a low antioxidant status may be the common mechanism
by which these factors operate.
This paper has focused on CHD in Britain but the conclusions
may be equally applicable to other diseases and other countries (including
Australia) where large variations in risk exist25,29.
Many correlations have featured in this paper. Correlations
are merely sign-posts pointing to possible research directions. More
basic research is required to understand how the antioxidants function
in health and disease on a molecular and cellular level. Novel approaches
are required to reliably measure antioxidant status and oxidative
stress. If these can distinguish between high- and low-risk groups
(eg smokers versus non-smokers) they can serve as clinical end-points
to test the efficacy of preventative and therapeutic regimens and
may also be useful as diagnostic tools to assess disease risk in individuals30.
Ways of screening foods and food additives for anti- and pro-oxidant
activity also need to be developed. Research programmes like that
initiated by the MAFF which bring together researchers, industry,
and government, will build a solid foundation of science on which
to base future dietary recommendations.
References
- Halliwell B, Gutteridge JMC. Free Radicals in Biology
and Medicine. Oxford: Oxford University Press; 1989.
- World health statistics annual. Geneva: WHO;1992.
- Roberts DCK. Dietary factors in the fall in coronary
heart disease mortality. Prostaglandins, leukotrienes and essential
fatty acids 1991;44:97-101.
- Smith WCS, Tunstall-Pedoe H, Crombie IK, Tavendale
R. Concomitants of excess coronary deaths - major risk factor and
lifestyle findings from 10,359 men and women in the Scottish Heart
Health Study. Scot Med J 1989;34:550555.
- Tunstall-Pedoe H, Smith WCS, Crombie IK,, Tavendale
R. Coronary risk factor and lifestyle variation across Scotland:
results from the Scottish Heart Health Study. Scot Med J 1989;34:556-560.
- Hargreaves AD, Logan RL, Thomson M, Elton RA, Oliver
MF, Riemersma RA. Total cholesterol, low density lipoprotein cholesterol
and high density lipoprotein cholesterol and coronary heart disease
in Scotland. Brit MedJ 1991;303:678-681.
- James WPT, Duthie GG, Wahle KWJ. The Mediterranean
diet: protective or simply non-toxic? Eur J Clin Nutr 1989;43:31-41.
- Riemersma RA, Oliver MF, Elton RA, Alfthan G, Vartiainen
E, Sale M, Rubba P, Mancini M, Georgi H, Vuilleumier J-P, Gey KF.
Plasma antioxidants and coronary heart disease: vitamins C and E,
and selenium. Eur J Clin Nutr 1990;44:143-150.
- Sauberlich HE. Vitamin C status: methods and findings.
Ann NY Acad Sci 1975; 258:438-450.
- Statters DJ, Asokan VS, Littlewood SM, Snape J.
Carcinoma of the caecum in a scorbutic patient. Brit J Clin Pract
1990;44:738-740.
- Tavendale R, Lee AJ, Smith WCS, Tunstall-Pedoe
H. Adipose tissue fatty acids in Scottish men and women: results
from the Scottish Heart Health Study. Atherosclerosis 1992;94:161-169.
- Riemersma RA, Wood DA, Butler S, Elton RA, Oliver
MF, Salo M, Nikari T, Vartiainen E, Puska P, Gey F, Rubba P, Mancini
M, Fidanza F. Adipose tissue linoleic acid and coronary heart diseases.
A report of surveys in Scotland, Finland and Italy. Br Med J 1986;292:14231427.
- Wood DA, Riemersma RA, Butler S, Thomson M, Macintyre
C, Elton RA, Oliver MF. Linoleic and eicosapentaenoic acids in adipose
tissue and platelets and risk of coronary heart disease. Lancet
1987;i:177-183.
- Katan MB, van Birgelen A, Deslypere JP, Penders
M, van Staveren WA. Biological markers of dietary intake with emphasis
on fatty acids. In: Kok FJ, van't Veer P, eds. Biomarkers of Dietary
Exposure. Smith-Gordon 1991; 37-49.
- Committee on Medical Aspects of Food Policy. 41
Dietary ? reference values for food energy and nutrients for the
United Kingdom. Department of Health, London: HMSO;1991.
- Bolton-Smith C, Woodward M, Tunstall-Pedoe H. The
Scottish Heart Health Study. Dietary intake by food frequency questionnaire
and odds ratios for coronary heart disease risk. II. The antioxidant
vitamins and fibre. Eur J Clin Nutr 1992;46:85-93.
- Yarnell JWG, Milbank J, Walker CL, Fehily AM, Hayes
28 TM. Determinants of high density lipoprotein and total cholesterol
in women. J Epidemiol Comm Health 1982;36:167-171.
- Yarnell JWG, Fehily AM, Milbank J, Sweetnam PM,
Walker CL. A short dietary questionnaire for use in an epidemiological
survey: comparison with weighed dietary records. Hum Nutr Appl Nutr
1983;37A:103-112.
- Bingham SA. Limitations of the various methods
for collecting dietary intake data. Ann Nutr Metab 1991;35:117-127.
- Anonymous (editorial). Dietary fibre: importance
of function as well as amount. Lancet 1992;340:1133-1134.
- Bolton-Smith C, Smith WCS, Woodward M, Tunstall
Pedoe H. Nutrient intakes of different social-class groups: results
from the Scottish Heart Health Study (SHHS). Brit J Nutr 1991;65:321-335.
- Gregory J, Foster K, Tyler H, Wiseman M. The Dietary
and Nutritional Survey of British Adults. London: HMSO; 1990.
- Duthie GG, Arthur JR, James WPT. Effects of smoking
and vitamin E on blood antioxidant status. Am J Clin Nutr 1991;53:1061S-1063S.
- Church DF, Pryor WA. Free-radical chemistry of
cigarette smoke and its toxicological implications. Environ Health
Perspect 1985;64:111-126.
- Marmot MG, Shipley MJ, Rose G. Inequalities in
death-specific explanations of a general pattern. Lancet 1984;1:1003-1006.
- Crombie IK, Kenicer MB, Smith WCS, Tunstall-Pedoe
HD. Unemployment, socio-environmental factors, and coronary heart
disease in Scotland. Brit Heart J 1989;61:172-177.
- Rosenman RH. Diet in haste; repent in leisure.
The Biochemist 1992;14:6-10.
- Carstairs V, Morris R. Deprivation: explaining
differences in mortality between Scotland and England and Wales.
Brit Med J 1989;299:886-889.
- Auckland, Newcastle and Perth Monica Centres. Risk-factor
levels and mortality of ischaemic heart disease in three Australasian
centres. Med J Aust 1988;148:61-65.
- Brown AJ. Oxidatively-modified lipoproteins in
coronary heart disease: novel approaches to the measurement of lipid
peroxidation in vivo. British Nutrition Foundation Bulletin 1992;
17 (suppl 1):49-64.

Copyright © 1993 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
to the top
|