Asia Pacific J Clin Nutr (1992) 1, 207-210

Insulin-like growth factor-I and
fast growth-hormone levels in mild and moderately malnourished children
Wan Mohamud Wan Nazaimoona
PhD, Ali Osmanb MPH, Mee Lian Nga PhD,
Tean Tune TanC MRCP, Loo Ling WUd MRCP, Othman SakinahC MD and Abdul Kadir KhalidC
PhD, FRACP.
Department(s) of aBiochemistry,
bPublic Health, cMedicine
and dPediatrics, Faculty of Medicine, Universiti
Kebangsaan Malaysia, Jin. Raja Muda Abd. Aziz, 50300 Kuala Lumpur,
Malaysia.
Insulin-like growth factor-I (IGF-I)and fasting growth
hormone levels were measured in a group of 255 children (163 males
and 92 females. age ranged 6-17 years) of varying pubertal development
and body mass index (BMI); well-nourished (BMI> 18). mildly-malnourished
(BMI = 15-18) and moderately-malnourished (BMI<15). In well-nourished
children IGF-I levels increased significantly (P = 0.02) with pubertal
development. where girls at Tanner 5 had significantly higher (p =
0.03) IGF-I levels than the boys. Whilst there was no change in fasting
GH levels with nutritional status, IGF-I levels of prepubertal boys
and girls decreased significantly with BMI (P<0.001 and P = 0.01
respectively). Hence. measurement of IGF-I levels is a sensitive biochemical
index in the assessment of mild and moderate form of malnutrition
in prepubertal children.
Introduction
Growth hormone (GH) mediates its growth-promoting
effect via a group of peptides known as somatomedin-C or insulin-like
growth factor-I (IGF-I)1. Generally, short stature is caused
by the lack of GH2, although in Laron-type dwarfism,
it is due to IGF-I deficiency3. Growth retardation
also occurs in children with protein-energy malnutrition (PEM), where
IGF-I levels were found to be low1,4,5 and GH were reported
to be elevated4,6-8.
Compared to other biological markers such as albumin
and transferrin, IGF-I was reported to be very sensitive to changes
in nutritional status and was recommended as a potential biological
marker in the assessment of chronic malnutrition such as kwashiorkor
and marasmus5,9-12. However, as such severe conditions
are rarely seen in Malaysia, we undertook this study to evaluate the
sensitivity of IGF-I and GH measurements in less severe forms of malnutrition,
and to assess their usefulness as biochemical indicators of nutritional
status.
Patients
and methods
A total of 255 children (163 males and 92 females,
age 6-17 years) from three rural villages were studied. Their pubertal
development was determined by the method of Tanner, whereby 143 children
were prepubertal (scrotum/breast stage 1) and 112 had already achieved
puberty (Tanner stages 2 to 5). These children were also grouped into
three groups according to their body mass index (BMI); well-nourished
(BMI>18), mildly-malnourished (BMI = 15-18) and moderately-malnourished
(BMI<15) (Table 1). Informed consent was obtained from all subjects
and their parents.
Table 1. Characteristics of the children in
the study.
| |
|
Pubertal development |
| |
|
Prepubertal |
Pubertal |
| Children |
Total |
BMI<15 |
BMI 15-18 |
BMI>18 |
BMI<15 |
BMI 14-18 |
BMI>18 |
| Boy |
163 |
51 |
24 |
13 |
10 |
43 |
22 |
| Girl |
92 |
33 |
16 |
6 |
0 |
4 |
33 |
| Together |
255 |
84 |
40 |
19 |
10 |
47 |
55 |
After an overnight fast, 5 ml of blood was obtained
by venepuncture, 2.5 ml being immediately transferred into an EDTA
tube and the remainder allowed to clot in a plain tube. All plasmas
and sera were separated immediately and stored frozen at -20°C until
assayed.
Plasma IGF-I levels were measured by SM-C RIA kit
from Nichols Institute, San Juan, Capistrana. CA. USA. Intra-assay
coefficient of variations (CVs) at concentrations of 0.5, 0.82 and
1.24 U/ml were 12.0, 9.0 and 7.4% respectively, whilst the respective
inter-assay CVs were 12.2, 10.0 and 8% .
Serum samples were assayed for GH using our own in-house
enzyme-linked immunoabsorbent assay13. Intra- and inter-assay
CVs at concentrations of 3.4-55.7 mIU/I were all within 10%. GH reference
standard used was I.S. 80/505 and minimal detectable concentration
of the assay was 0.4 mIU/I. As far as possible. samples within each
group were assayed in a single assay.
Statistical
Analysis
Differences between groups were analysed by nonparametric
Kruskal-Wallis and Wilcoxon Rank Sum tests. A P value of <0.05
was regarded as significant.
Results
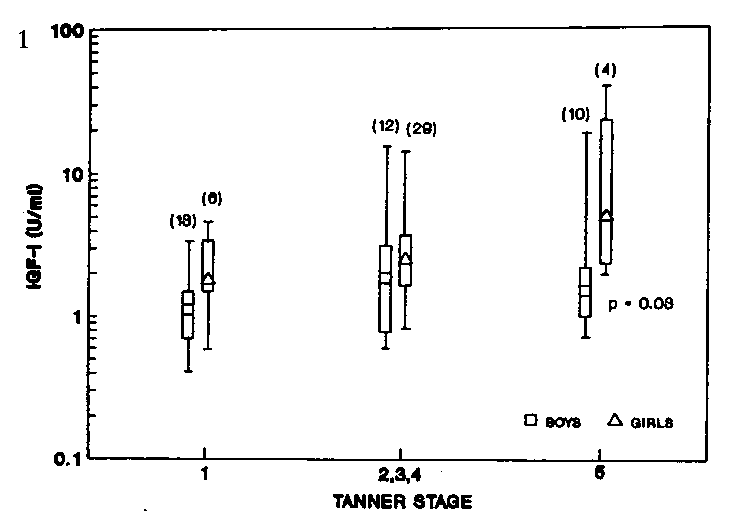
Plasma IGF-I levels in 74 normal. well-nourished children
(BMI>18) (35 males and 39 females) at different Tanner stages arc
shown in Fig 1. IGF-I levels increased significantly (P = 0.02) with
pubertal development. Sex-difference in IGF-I levels was observed
only at Tanner stage 5. Girls in this group had significantly higher
(P = 0.03) IGF-I levels than the boys (Fig 1).
Figure 1. Plasma insulin-like growth factor-I
(IGF-I) of normal boys and girls at different Tanner stages. 95% confidence
limits and interquartile ranges are represented by vertical columns
and bars respectively. Numbers above bars represent number of children
studied.
Changes in nutritional status (based on BMI) were
found to influence only the IGF-I levels of the prepubertal group
(Fig 2). Plasma IGF-I levels decreased significantly in the mildly
and moderately-malnourished boys and girls (P<0.001 and P =.0.01
respectively). Median IGF-I levels of the moderately-malnourished
boys and girls (0.5 and 0.78 U/ml respectively) were significantly
lower (P = 0.004 and P = 0.03 respectively) than the respective sex-matched
well-nourished group (1.1 and 1.8 U/ml respectively) (Fig 2). On the
other hand, there was no significant difference in fasting GH levels
between the well-nourished, mildly- and moderately-malnourished prepubertal
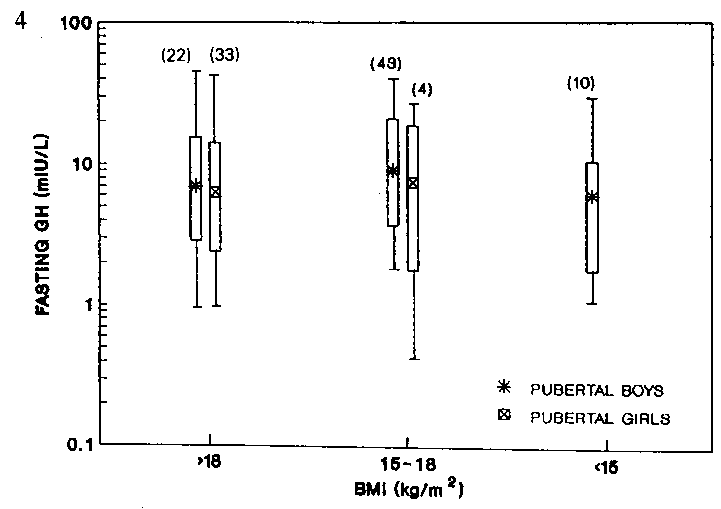
children (Fig 3) or pubertal boys (Fig 4). We were however, unable
to draw any conclusion on the fasting GH levels of the pubertal girls.
as there were insufficient data in the mildly- and moderately-malnourished
groups (n = 4 and n = 0 respectively) (Fig 4).
Figure 2. Plasma insulin-like growth factor-l
(IGF-I) of prepubertal boys and girls at different nutritional status.
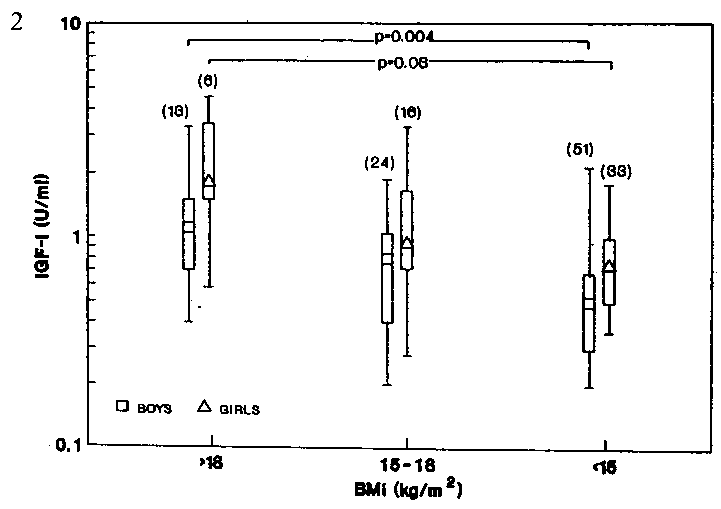
Figure 3. Fasting growth hormone of prepubertal
boys and girls of different nutritional status.
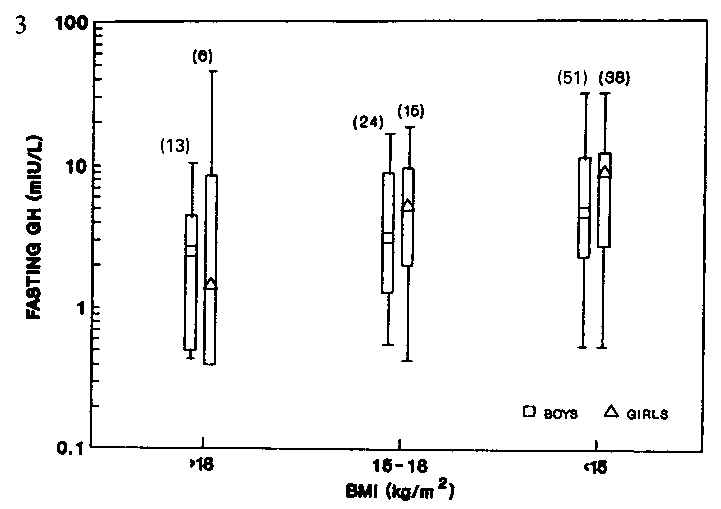
Figure 4. Fasting growth hormone of pubertal
boys and girls of different nutritional status.
Discussion
The pubertal increase in IGF-I levels of normal children
seen in this study is comparable to that reported by others14-16.
Similarly to the report by Ruland et al.16, who found that
girls age 6-14 years had higher IGF-I levels than boys, we found that
sex-difference was most significant at Tanner 5 (P = 0.03). We suggest
that this was due to the influence of oestrogens17,18.
The various alterations in hormonal levels seen in
severe malnutrition are believed to be part of the endocrine adaptive
processes to conserve energy and protein metabolism in adverse conditions.
In this study, plasma IGF-I levels in prepubertal children were found
to decrease significantly with BMI. This finding is comparable to
that observed by others4,8. In contrast to previous
reports,4,6,8 we found that there was no significant difference
in fasting GH levels between the well-nourished, mildly and moderately
malnourished children. This is probably due to the less severe form
of malnutrition of our subjects, compared to other studies4,7,8
where the children were suffering from marasmus and kwashiorkor.
Hence, in agreement with several previous studies,5,9,11,12
we have showed that measurement of IGF-I concentrations is indeed
sensitive to slight changes in nutritional status and thus is a better
biochemical index than GH in the assessment of growth retardation
due to malnutrition.
References
- Phillips LS. Vassilopoulou-Scllin R. Nutritional
regulation of somatomedin. Am J Clin Nutr 1979: 32: 1082.
- Daughaday WH. Growth hormone and the somatomedins.
In: Daughaday WH. cd. Endocrine control of growth. New York: Elsevier.
1981; 1.
- Laron. Z. Petzelan A. Karp M. Kowaldlo-Silbergeld
A. Daughaday WH. Administration of growth hormone to patients with
familial dwarfism with high plasma immunoreactive growth hormone:
measurement of sulfation factor. metabolic and linear growth responses.
J Clin Endocrinol Metab 1971; 33:332.
- Hintz RL. Suskind R. Amatayakul K. Thananykul O.
Olson R. Plasma somatomedin and growth hormone values in children
with protein-calorie malnutrition. J Pediatr IY78:92:153.
- Unterman TG. Vazquez RM. Slas AJ. Martyn PA. Phillips
PA. Nutrition and somatomedin. XIII. Usefulness of somatomedin-C
in nutritional assessment. Am J Med 1985; 78:228.
- Beas F. Contreras 1. Maccioni A. Arena S. Growth
hormone in infant malnutrition: the arginine test in marasmus and
kwashiorkor. Br J Nutr 1971; 26: 169.
- Lunn PG. Whitehead RG. Hay RW. Baker BA. Progressive
changes in serum cortisol. insulin and growth hormone concentrations
and their relationship to the distorted amino acid pattern during
the development of kwashiorkor. Br J Nutr 1973; 29:399.
- Smith IF. Latham MC. Azubuike JA. Butler WR. Phillips
LS, Pond WG. Enwonwu SO. Blood plasma levels of cortisol. insulin.
growth hormones and somatomedin in children with marasmus. kwashiorkor.
and intermediate forms of protein-energy malnutrition. Proc Soc
Exp Biol Med 1981: 167:607.
- Clemmons DR, Underwood LE. Dickerson RN. Brown
RO, Hak LJ, MacPhee RD. Heizer WD. Use of plasma somatomedin-C/insulin-like
growth factor I measurements to monitor the response to nutritional
repletion in malnourished patients. Am J Clin Nutr 1985; 41:191.
- Phillips LS. Nutrition. somatomedins and the brain.
Metabolism 1986; 35:78.
- Minuto F. Barreca A. Adami GF. Fortini P. Del Monte
P. Cella F. Scopinaro N. Giordano G. Insulin-like growth factor-I
in human malnutrition: relationship with some body composition and
nutritional parameters. J Parenter Enteral Nutr 1989; 13:392-6.
- Bouhaddioui L. Brun JF, Jacquemin JC. Bouhaddioui
N. Kabbaj K. Orsetti A. Immunoreactive somatomedin C in children
from Morocco: a biological marker of nutritional growth retardation?
Biomed. Pharmacother 1989; 43:59.
- Ng ML. Goh KH, Wan Nazaimoon WM. Thean ETT. Khalid
BAK. Non-isotopic in-house ELISA as an alternative to RWI RMA for
measurement of pituitary peptide hormones. IAEA/WHO International
Symposium on RIA and related procedures. Perspectives in Developing
Countries. 26-30 Aug 1991, Vienna. Austria. IAEA-SM324/24: 109.
- Bala RM, Lopatka J, Leung A. McCoy E. McArthur
RG. Serum immunoreactive somatomedin levels in normal adults. pregnant
women at term, children at various ages and children with constitutionally
delayed growth. J Clin Endocrinol Metab 1981; 52:508.
- Luna AM. Wilson DM. Wibbelsman CJ. Brown RC. Nayashima
RJ. Hint RL. and Rosenfeld RG. Somatomedins in adolescence: a cross
sectional study of the effect of puberty on plasma insulin-like
growth factor I and II levels. J Clin Endocrinol Metab 1983; 57:268.
- Ruland A. Heinrich U. Hartmann K. Schonberg D.
Scrum IGF-I levels during childhood and adolescence. Acta Paediatr
Scand [Suppl] 1988: 343:238.
- Cuttler L. Van Vliet G. Conte FA. Kaplan SL. Grumbach
MM. Somatomedin-C levels in children and adolescents with gonadal
dysgenesis: differences from age matched normal females and effect
of chronic estrogen replacement therapy. J Clin Endocrinol Metab
1985: 60: 1087.
- Rosenfield RL. Furlanetto R. Physiologic testosterone
and estradiol induction of puberty increases plasma somatomedin-C.
J Pediatr 1985; 107:415.
- Brasel JA. Endocrine adaptation to malnutrition.
Pediatr Rcs 1980: 14:415.
- Laditen AA. Hormonal changes in severely malnourished
children. African J Med Sci 1983: 12: 125.
Insulin-like growth factor-I and
fast growth-hormone levels in mild and moderately malnourished children
Wan Mohamud Wan Nazaimoon, Ali Osman,
Mee Lian Ng. Tean Tune Tan, Loo Ling Wu, Othman Sakinah and Abdul
Kadir Khalid
Asia Pacific Journal of Clinical
Nutrition 1992; 1: 207-210
ARAS FAKTOR PERTUMBUHAN MENYERUPAI
INSULIN I (IGF-I) DAN HORMON PERTUMBUHAN BERPUASA PADA KANAK-KANAK
YANG MENGALAMI KEKURANGAN GIZI YANG RENDAH DAN SEDERHANA
Aras faktor pertumbuhan menyerupai insulin I (IGF-I)
dan hormon pertumbuhan berpuasa dihitung pada 273 orang kanak-kanak
(186 orang kanak-kanak lelaki dan 87 orang kanak-kanak perempuan.
yang berusia sekitar 6-17 tahun) dari pelbagai peringkat pubertas
dan indeks massa tubuh (BMI); tanpa kekurangan gizi (BMI>18). kekurangan
gizi yang rendah (BMI = 15-18) dan kekurangan gizi yang sederhana
(BMI<15). Pada kanak-kanak yang tidak mengalami kekurangan gizi.
aras IGF-I meningkat secara signifikans (P=0.02) bersama peringkat
pubertas, yang mana kanak-kanak perempuan pada Tanner 5 mempunyai
aras IGF-I yang signifikans lebih ketara dari kanak-kanak lelaki (P=0.03).
Sungguhpun tiada perubahan aras hormon pertumbuhan berbanding status
gizi, paras IGF-I pada kanak-kanak lelaki dan perempuan prepubertas
menurun secara signifikans bersama-sama BMI (masing-masing P<0.001
dan P=0.01). Oleh itu, perhitungan aras IGF-I merupakan indeks biokiminia
yang sensitif untuk menilai kekurangan gizi yang rendah dan sederhana
pada kanak-kanak prapubertas.

Copyright © 1992 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
to the top