Asia Pacific J Clin Nutr (1992)1, 175-182

Promotion of healing by orally
administered glutamine in elemental diet after small intestinal injury by X-ray radiation
Takuzo Nambu MD, Tadao Bamba MD, PhD, and Shiro Hosoda
MD, PhD
Second Department of Internal Medicine,
Shiga University of Medical Science, Otsu, Shiga, Japan.
Glutamine was administered orally to rats with damaged
small intestinal mucosa as the result of injury by X-ray radiation
at 10 Gy to the abdomen. The healing effects of glutamine on the
injured mucosa were studied serially from the day of radiation (Day
0) to Day 4. The rats received two types of isocaloric elemental
diet, Gln( + ) containing 2% glutamine and Gln( - ) containing no
glutamine, by paired feeding.
From Day 2 to Day 4, the wet weight, protein content,
and DNA content of the jejunal mucosa were significantly greater
in the Gln(+) than in the Gln(-) group. On Day 3, when the damage
of the intestinal mucosa was the severest, the crypt cell production
rate in the jejunum was significantly higher in the Gln(+) than
in the Gln(-) group. The permeability of the intestinal mucosa to
51CrEDTA, administered to the rat stomach through an
oro-gastric tube, remained significantly lower in the Gln( + ) group
. Light microscopic findings showed that oedema in the lamina propria
mucosae of jejunum and fusion of jejunal villi were milder in the
Gln(+) group on Day 4. when the mucosal mass began to recover. The
arterial and portal blood glutamine concentration, and glutamine
extraction by the gut from arterial blood and phosphate-dependent
glutaminase activity in the jejunal mucosa, were higher in the Gln(+)
than in the Gln(--) group. Ornithine decarboxylase activity was
increased in both the jejunum and the ileum from Day 3, but no difference
was observed between the two groups.
These findings suggest that, after X-ray radiation
injury of the intestinal mucosa, the oral administration of the
elemental diet containing 2% glutamine improved glutamine metabolism
of the body, promoted the proliferation of jejunal epithelium, accelerated
the recovery of the mucosal mass and the morphology of villi, and
then contributed to maintaining the barrier function of the intestine
from an early stage after the injury.
Introduction
Glutamine has not been considered important as a component
of nutritional preparations, because it is a non-essential amino acid,
has a low solubility, and is unstable in aqueous solution forming
pyroglutamate and ammonia1. Recent studies, however, have
disclosed that enterocytes play an important role in metabolism of
amino acids, especially glutamine, and glutamine is the primary source
of energy for the enterocytes2-4. It has also
been reported that glutamine requirement increases when the intestinal
mucosa is damaged5-6. These observations have led to increased
attention to glutamine as a nutrient for the intestine with injured
or atrophic mucosa7-10. Recently, there have been many
reports that oral administration of glutamine from a few days before
intestinal injury had prophylactic effects11-13. There
were also some reports that indicated that oral administration of
glutamine after injury promoted for the intestinal mucosal repair14.
After the rat intestinal mucosa had been damaged by X-ray radiation,
Klimberg et al.15 orally administered a preparation containing
3% glutamine as the only amino acid and showed the usefulness of the
glutamine by evaluation of the mucosal mass and glutamine metabolism
on Day 8. However, there are few studies on the effects of glutamine,
orally administered after injury, on the early healing process in
injured intestinal mucosa and which considering the amino acids balance.
In this study, therefore, effects of glutamine in
the elemental diet administered after injury on the small intestinal
repairing process were examined by mucosal mass, crypt cell proliferation,
glutaminase activity, and permeability to 51Cr-EDTA.
Furthermore, ornithine decarboxylase (ODC) is a key
enzyme for cell proliferation, and its activity is known to increase
before cell proliferation16 ,17. The activity of this enzyme,
moreover, is reported to increase during the mucosal repair after
intestinal damage18,19 . Thus, the changes of ODC activity
in the small intestinal mucosa were also measured serially.
Materials
and methods
Wistar male rats, weighing 240 270 g (Clea Japan Co.,
Tokyo, Japan), were housed in wire-bottom cages and acclimated for
at least three days. During this period, the animals were given common
food pellets for rats and water ad libitum.
Experiment
1: Mucosal parameters and blood glutamine concentratlon
Changes in the intestinal mucosal mass, polyamine
synthesis, histological findings, glutaminase activity, and blood
glutamine concentration were measured in 66 rats before and after
X-ray radiation to the abdomen.
First, 12 rats were divided at random into two groups
given a glutamine-free diet (Gln [-] group) and a group given a glutamine-containing
diet (Gln [+] group). After an overnight fast, the animals were anaesthetized
by intraperitoneal administration of pentobarbital (30 mg/ kg body
weight) between 10:00 and 12:00, weighed and radiated with X-rays
in a single dose of 10 Gy. The rats were fixed in the supine position
on the X-ray radiator (MBR-1520R, Hitachi), covered with a lead plate
3 cm in thickness except for the abdomen from the xyphoid process
to the pubis (field, 9 x 6 cm2), and radiated at a source-skin distance
of 50 cm.
The rats were then caged individually and given Gln(-)
or Gln(+) diet. The food was given ad libitum, but the daily food
intake of the two groups was equalized by paired feeding.
The food was prepared by eliminating or adding glutamine
from the elemental diet Elentalâ (Ajinomoto Co., Tokyo, Japan).
In order to equalize the weight of total amino acids of Gln(-) to
that of Gln(+), the contents of other amino acids were increased in
Gln(-) and were reduced in Gln(+) (Table 1). They were given to the
rats after being dissolved at 1 kcal/ml. The glutamine concentration
of Gln(+) preparation in the diluted solution was 2.0%.
Table 1. Composition of diet formulas (mg).
| Amino acids |
Elentalâ a |
Gin(-)b |
Gin(+)c |
| Gln |
2415 |
0 |
7500 |
| Ile |
803 |
941 |
512 |
| Leu |
1124 |
1318 |
716 |
| Lys |
888 |
1041 |
566 |
| Met |
810 |
950 |
516 |
| Phe |
1089 |
1277 |
696 |
| Thr |
654 |
767 |
417 |
| Trp |
189 |
222 |
120 |
| Val |
876 |
1027 |
558 |
| His |
463 |
543 |
295 |
| Arg |
1163 |
1363 |
741 |
| Ala |
1124 |
1318 |
716 |
| Asp |
1823 |
2137 |
1161 |
| Gly |
631 |
740 |
402 |
| Pro |
788 |
924 |
502 |
| Ser |
1449 |
1699 |
923 |
| Tyr |
138 |
162 |
88 |
| Total amino acid (g) |
16.427 |
16.427 |
16.427 |
| Dextrin (g) |
79.37 |
79.37 |
79.37 |
| Soy bean oil (g) |
0.636 |
0.636 |
0.636 |
| Others (g) |
3.567 |
3.567 |
3.567 |
| Total (g) |
100.000 |
100.000 |
100.000 |
a Clinically used elemental diet.
bGln(--) contained no glutamine. The content of other amino
acids was more than that of Elental and the ratio was 1:1.172.
cGln(+) contained 2% glutamine when it was diluted to 1
kcal/ml solution. Other amino acids were less than that of Elental
(1:0.637).
At night on the day of radiation, the rats were anaesthetized
intraperitoneally with pentobarbital, the abdominal wall was incised
after body weight measurement, a 21-gauge catheter was inserted into
the portal vein and 2-3 ml of the portal blood was collected. Blood
was also sampled from the abdominal aorta. These blood samples were
deproteinated by adding the same volume of 5% sulfosalicylic acid,
and mixing sufficiently, and centrifuged at 30 000 g at 4°C for 15
minutes. The supernatant was stored at -20°C and assayed later for
glutamine by automated high performance liquid chromatography (L-8500,
Hitachi, Tokyo, Japan)20. Glutamine extraction from artery
by the gut was calculated by the following equation15:
Ext=(A-P)/A x 100
EXT:Gut glutamine extraction (%).
A:Arterial glutamine concentration.
P:Portal glutamine concentration.
The rats were then decapitated, the small intestine
from Treitz's ligament to the terminal ileum was resected, rinsed
with phosphate buffered saline (pH7.6) at 4°C, and suspended with
a 10-g weight. A 10-cm jejunal segment from 5 cm to 15 cm anal from
Treitz's ligament and a 10-cm ileal segment from 10 cm to 20
cm oral from the terminal ileum were cut, incised longitudinally on
an ice-cooled plate, and the mucosa was scraped with a glass slide.
After measurement of the wet weight of the mucosa, it was homogenized
at 4°C for 30 seconds with 10 ml phosphate buffered saline (pH7.2)
containing 0.1 mM pyridoxal-5'-phosphate and 5 mM dithiothreitol with
an ultra-disperser (LK-22, Yamato Scientific Co., Tokyo, Japan). A
part of the homogenate was used for determination of the ODC activity
which was measured by the release of 14CO2 from
L-(1-14C)-ornithine (American Radiolabeled Chemicals Inc.,
St. Louis, USA)18, and the other part was mixed with the
same volume of 10% trichloroacetic acid, stirred, and centrifuged
at 30 000 g for 15 minutes, and the supernatant was stored at -70°C
for the assay of polyamines by HPLC21. The remaining homogenate
was stored at -20°C for determination of the protein content by the
method of Lowry et al.22 and the DNA content by fluorometric
method23.
Next, 10-cm segments were collected from 15 cm to
25 cm anal from Treitz's ligament and from 20 cm to 30 cm oral from
terminal ileum. The mucosa was scraped, homogenized with 5 ml of 125
mM potassium phosphate buffer (pH7.6) containing 330 mM sucrose and
2 mM dithiothreitol by 20 strokes of a motor-driven Teflon-glass homogenizer,
and assayed for the phosphate-dependent glutaminase activity24.
Segments (1 cm) were collected from the jejunal and
ileal stumps, immersed with 10% buffered formaldehyde solution, and
examined histologically under a light microscope with hematoxylin
-eosin stain.
The jejunal and ileal mucosa was collected by the
same method from the two groups (n=6 each) on four consecutive days
from the day after X-ray radiation (Day 1-4). Sampling was done similarly
in six rats on the day before X-ray radiation (Day-1). All samplings
were done between 21:00 and 23:00 each day while confirming that the
food had arrived at the stomach and the small intestine, to evaluate
the effect of oral food intake on the intestinal mucosal ODC activity
under the same conditions. Polyamines were assayed in the samples
only on the day when ODC activity was elevated.
Experiment
2: Crypt cell production rate (CCPR)
Twenty rats were divided into two groups after X-ray
radiation, and were maintained with Gln(-) and Gln(+) diet as in Experiment
1. On Day 3, the animals were anaesthetized with diethylether, injected
intraperitoneally with vincristine sulfate (Shionogi Pharmaceutical
Inc., Osaka, Japan) at 1.0 mg/kg body weight, and two animals in each
group were killed by decapitation after 30, 50, 70, 90, and 110 minutes.
Jejunal and ileal segments were collected from the same sites as in
Experiment 1, and were immersed in Carnoy's fixing fluid. These samples
were stained with Schiff's reagent, crypts were cut one by one under
a stereoscopic microscope, and ten crypts in each intestinal segment
were picked up. The number of metaphase cells per crypt was counted
under a light microscope, and CCPR was calculated25
Experiment
3: Intestinal permeability
Twelve rats were divided into two groups after X-ray
radiation, and were fed with Gln(--) or Gln(+) diet. On Day 3, the
animals were anaesthetized with diethylether, and 2 ml of physiologic
saline containing 37 kBq 51Cr-EDTA (NEN Research Product
Co., Boston, USA) was infused into the rat stomach through an oro-gastric
tube. The rats were thereafter housed individually in metabolic cages,
being allowed to have free access to water. The radioactivity in the
urine pooled for six hours was counted with a gamma counter, and the
permeability of the intestine to 51Cr-EDTA was calculated26.
Statistical
analysis
The statistical analyses were made using the
Stat Flex statistical program (Nankodo Co., Tokyo, Japan). The data
were expressed as the means± SEM and compared between the two groups
by t-test at the significance level of P<0.05.
Result
Experiment
1: Mucosal parameters and blood glutamine concentration
All rats survived after X-ray radiation. The food
intake of the rats was 12-38 ml/rat/day (the mean± SEM for 4 days was 23.8± 3.3 ml/rat/day) and was lowest during Day 2-3 (Table 2). No significant
difference was observed in body weight changes between the Gln(--)
and the Gln(+) groups.
Table 2. Oral intake and body weight change.
| Oral intakea |
| Date |
Day -1~0 |
0~1 |
1~2 |
2~3 |
3~4 |
Day0~4 |
| Intake(ml/day/rat) |
Starved |
38± 5 |
27± 3 |
12± 3 |
18± 3 |
23.8± 3.3 |
| Body weight change from Day -1b |
| Date |
Day 0 |
1 |
2 |
3 |
4 |
|
| Gin(-)(g) |
-5± 1 |
-19± 1 |
-36± 2 |
-58± 2 |
-71± 2 |
|
| Gin(+)(g) |
-8± 1 |
-21± 1 |
-35± 2 |
-52± 3 |
-64± 2 |
|
a Measurement of six pairs of rats killed on Day 4
(mean± SEM). b Measurement of six rats in each fed group killed on Day 4 (mean± SEM).
The wet weight of the mucosa was lowest on Day 3 in
the jejunum and ileum in both groups and tended to recover on Day
4. It was significantly higher in the Gln(+) than in the Gln(-) group
in the jejunum on Day 2, 3, and 4. Changes in the protein content
were similar to those of the wet weight of the mucosa and were significantly
higher in the Gln(+) than in the Gln(-) group in the jejunum on Day
2,3, and 4. The DNA content also showed similar changes (Figs 1 and
2).
Figure 1. Wet weight, protein content, and
DNA content in the jejunal mucosa. X-ray radiation was performed on
Day 0.
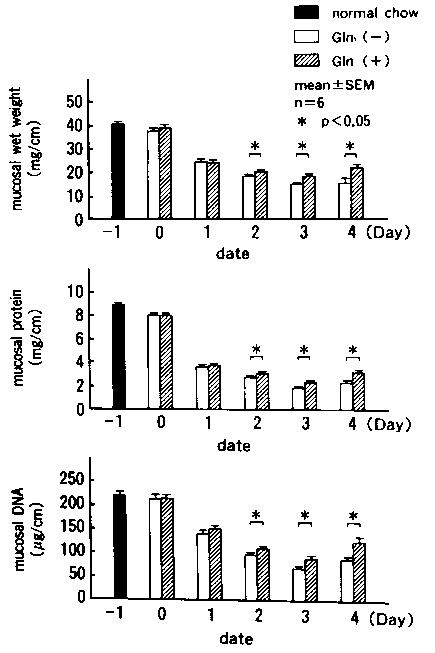
Figure 2. Wet weight, protein content, and
DNA content in the ileal mucosa.

By light microscopy on Day 4, when the damaged mucosa
began to recover, the jejunum in the Gln(-) group showed marked oedema
and inflammatory cell infiltration in the lamina propria mucosae,
marked fusion of villi, and severe morphological abnormalities (Fig.
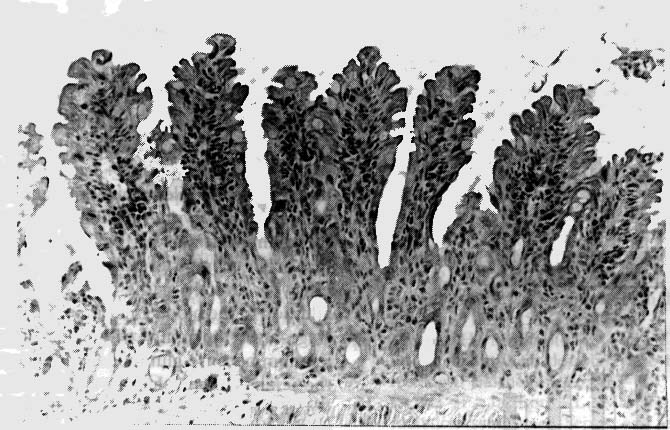
3). Such changes were present but milder in the Gln(+) group (Fig.
4). The morphological differences between two groups were also shown
in the jejunum on Day 2 and 3, but they were not so remarkable as
those on Day 4.
Figure 3. Light microscopic section of jejunum
from Gln(-) rat on Day 4 (haematoxylin-eosin stain, original magnification
x 200). Marked oedema and inflammatory cell infiltration in the lamina
propria mucosae, and marked fusion and deformity of the villi, are
shown.
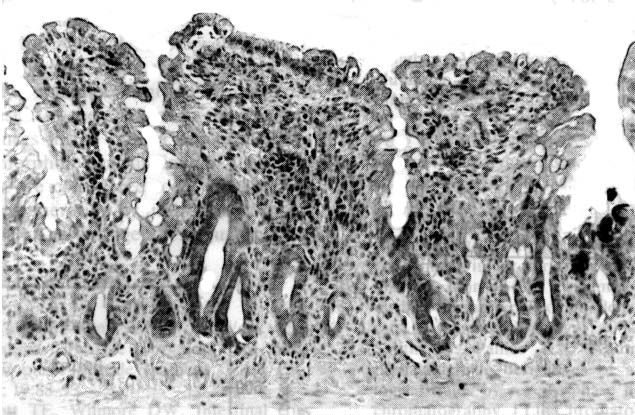
Figure 4. Light microscopic section of jejunum
from Gln(+) rat on Day 4 (haematoxylin-eosin stain, original magnification
x 200). Oedema, inflammatory cell infiltration in the lamina propria
mucosae, and fusion and deformity of villi are milder than those of
Gln(-).
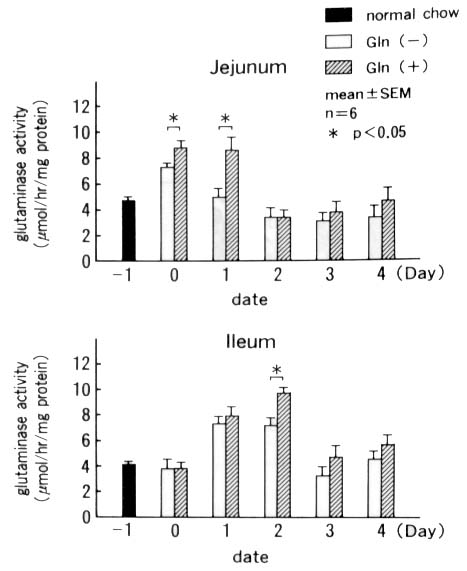
The phosphate-dependent glutaminase activity in the
intestinal mucosa was increased in the jejunum on Day 0 and Day 1
in both groups, suggesting the increased glutamine utilization, but
it was significantly higher in the Gln(+) than in the Gln(-) group.
In the ileum, the activity increased from Day 1 and became higher
in the Gln(+) than in the Gln(-) group on Day 2 (Fig. 5).
Figure 5. Phosphate-dependent glutaminase activities
in the jejunal and ileal mucosa.
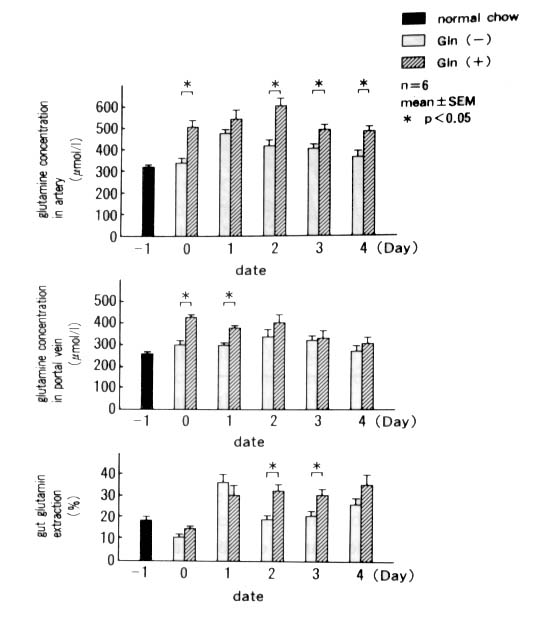
The arterial glutamine concentration was significantly
higher in the Gln(+) than in the Gln(-) group from Day 0 to Day 4
except Day 1. In the portal blood, the glutamine concentration was
significantly higher in the Gln(+) than in the Gln(-) group on Day
0 and 1. The glutamine extraction in the intestine was significantly
higher in the Gln(+) than in the Gln(-) group on Day 2 and 3 (Fig.
6).
Figure 6. Glutamine concentration in artery
and portal vein, and gut glutamine extraction. Gut glutamine extraction
(Ext) was calculated by following equation: Ext = (arterial Gln-portal
Gln)/arterial Glnxl00.
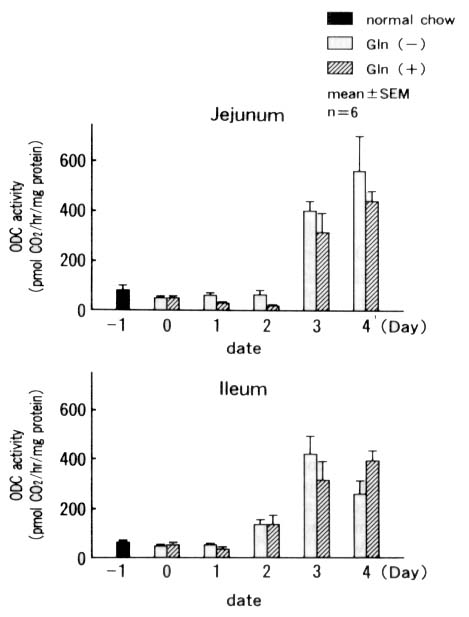
The ODC activity increased in both the jejunum and
ileum after Day 3, but no significant difference was observed between
the two groups (Fig. 7).
Figure 7. Ornithine decarboxylase (ODC) activities
in the jejunal and ileal mucosa.
Mucosal polyamine levels were determined in the jejunum
using samples on Day 3. No significant difference was observed in
the putrescine, spermidine, or spermine levels between the two groups
(Table 3).
Table 3. Polyamine content in the jejunal mucosa
on Day 3 (n mol/g protein).
| |
Putrescine |
Spermidine |
Spermine |
| Gln (-) |
849± 51 |
5324± 632 |
2379± 266 |
| Gln (+) |
654± 66 |
5034± 587 |
2273± 278 |
Mean± SEM, n=6. There were no significant differences between Gln(-) and Gln(+).
Experiment
2: CCPR
In the jejunum, CCPR was significantly higher in the
Gln(+) than in the Gln(-) group on Day 3, 22.8± 0.9 vs 14.4± 0.4 cells produced /crypt/h (mean± SEM, n=20 crypts, P<0.05), suggesting that crypt cell proliferation
was promoted by glutamine in the jejunum. No significant difference
was observed between the two groups in the ileum, ie 20.6± 0.9 vs 16.9± 0.8.
Experiment
3: Intestinal permeability
The permeability of the intestine to 51Cr-EDTA
was significantly lower in the Gln(+) than in the Gln(-) group on
Day 3, ie 3.5± 0.9 vs 9.5± 1.8 % (mean± SEM, n=6, P<0.05), suggesting that the glutamine administration kept
the permeability of the intestinal mucosa low even under the conditions
of the severest atrophy of the intestinal mucosa.
Discussion
In many earlier studies on the effects of oral glutamine
administration on injured intestinal mucosa, glutamine was replaced
with glycine in food given to animals of the control group. In this
study, however, we avoided replacing glutamine with glycine, which
has a strong ODC-inducing activity in the normal small intestine27,28,
because we also intended to study the ODC activity. Animals were fed
food obtained by modifying the composition of Elentalâ , a clinically used elemental diet, without
changing the total amino acids contents or increasing the contents
of particular amino acids other than glutamine. In our preliminary
study, the repairing effect of the elemental diet containing 0.6%
(Elentalâ ) and 1.0% glutamine on injured
intestinal mucosa was not so remarkable, and so, the diet containing
2% glutamine was used in this study.
In our preliminary examinations, the repairing effect
of oral glutamine on mildly injured intestinal mucosa by low dose
radiation at 2.5 Gy or 5.0 Gy had not been remarkable, and on the
other hand, the rats had died from Day 1 to Day 3 by high dose radiation
at 12.5 Gy or 15 Gy. Therefore, we settled the dose as 10 Gy.
Since the food was orally ingested by the rats, the
oral glutamine intake was decreased after X-ray radiation, so that
it might have not been sufficient to prevent degradation of muscle
protein and body weight losses associated with the release of endogenous
glutamine induced by injury of the intestine. However, the Gln(+)
diet had nutritional effects on the intestinal mucosa. The differences
of the mucosal parameters between the Gln(-) and the Gln( + ) group
suggested that orally administered 2% glutamine promoted repair of
the jejunal mucosa. It was also recognized because glutamine administration
was started after the injury that the differences were not due to
prophylactic effect of glutamine but a repairing effect by cell proliferation.
This effect was observed from Day 2, when the mucosa was damaged severely,
to Day 4, when signs of recovery began to appear. It was also confirmed
morphologically that glutamine markedly contributed to the repair
of the jejunal mucosa on Day 4. Recently, bacterial translocation
with injured mucosa of small and large intestine or with the mucosal
atrophy by total parenteral nutrition has attracted attention11,29.
The degree of bacterial translocation is not seemed to parallel with
that of the permeability to 51Cr-EDTA with a molecular
weight of 358. However, the permeability to 51Cr-EDTA is
considered to reflect an aspect of the barrier function of the intestinal
mucosa and it is also useful to evaluate the active stage of inflammatory
bowel disease30. In this study, the difference of the permeability
between the two groups indicated the usefulness of glutamine to prevent
the intestinal barrier function from the destruction. In addition,
the increased CCPR on Day 3 suggested that proliferation of crypt
cell and regeneration of the villous epithelium in the jejunum were
promoted by glutamine from the early stage when the damage of the
intestinal mucosa was severest.
Since arterial and portal blood flow were not measured
in this study, the true quantity of the glutamine extracted by the
gut was unknown. But the degrees of the extraction could be comparable
between the two groups4,15. The data of arterial glutamine
concentration and glutamine extraction showed that glutamine supply
from the blood as the fuel for the intestinal repair was increased
in the Gln(+) by comparison with the Gln(-) group. The activity of
phosphate-dependent glutaminase, which is the major enzyme involved
in the metabolism of glutamine, increased in the jejunum from Day
0 (12 hours after X-ray radiation and about four hours after the beginning
of oral ingestion of the food), suggesting that glutamine was utilized
in the jejunum mucosa from a very early stage after injury. However,
in spite of a high percentage of gut glutamine extraction of the Gln(+)
group on Day 2 and Day 3, the dates when the glutaminase showed higher
activities in the Gln(+) group were Day 0 and Day 1. There was a time
gap between the changes of the glutamine extraction and jejunal glutaminase
activity. Moreover, the glutaminase in the ileum also showed high
activity in the Gln(+) than in the Gln(-) group. If the arterial blood
was the primary source of glutamine, there would be no time gap, and
if so, the repair in the Gln(+) group might have also been promoted
in the ileum, where glutaminase activity was increased, however a
healing effect was notable only in the jejunum. It may be inferred
from these observations that orally administered glutamine produced
a repairing effect by directly acting on the intestinal epithelium
in addition to the blood glutamine supply, from a very early stage
after the injury, the effect being notable in the jejunum because
glutamine concentration was higher in the jejunal lumen than in the
ileal lumen. A study involving glutamine infusion through an ileal
fistula may be needed to confirm this hypothesis.
The ODC activity increased after Day 3, suggesting
cell proliferation in crypts. However, there was no significant difference
between the Gln( - ) and the Gln(+) groups or between the jejunum
and the ileum. In the jejunum, polyamines levels were not different
between the Gln(-) and the Gln(+) group. Enteral amino acids induce
ODC activity in the normal intestine, but the degree of induction
differs among the kinds of amino acids. Glutamine is one of those
with less ODCinducing activities28. In this study, it was
suspected that the changes of ODC activity by glutamine administration
were masked by the effect of the other amino acids than glutamine.
However, CCPR was significantly increased in the Gln(+) group. This
findings indicated that the proliferation of mucosal cells were promoted
by oral glutamine administration.
Conclusion
An elemental diet containing 2% glutamine was orally
administered to rats after mucosal injury was induced in the small
intestine by abdominal X-ray radiation at 10 Gy and serial changes
of the intestinal mucosa were studied. It was shown that radiation
injury was milder in the jejunum from Day 2 to Day 4 in the group
administered glutamine as the result of the changes in the mucosal
parameters, differences in the barrier function of the intestine,
and the morphological findings of villi. The effects of glutamine
are considered to be due to promotion of mucosal regeneration by utilization
of glutamine as gut fuel from an early stage after injury.
References
- Dimarchi RD. Tam JP, Kent SBH et al. Weak acid
catalyzed pyrrolidone carboxilic formation from glutamine during
solid phase peptide synthesis. Int J Pept Protein Res 1982; 19:88-93.
- Souba WW, Scott TE, Wilmore DW. Intestinal consumption
of intravenously administered fuels. J Parenter Enteral Nutr 1985;
9:18-22.
- Souba WW, Herskowitz K, Salloum RM et al. Gut glutamine
metabolism. J Parenter Enteral Nutr 1990; 14:45S-50S.
- Mc Anena OJ, Moore FA, Moore EE et al. Selective
uptake of glutamine in the gastrointestinal tract: confirmation
in a human study. Br J Surg 1991; 78:480-482.
- Smith RJ, Wilmore DW. Glutamine nutrition and requirements.
J Parenter Enteral Nutr 1990; 14:94S-99S.
- Salloum RH, Copeland EM, Souba WW. Brush border
transport of glutamine and other substrates during sepsis and endotoxemia.
Ann Surg 1991; 213:401-410.
- Hwnag TL, O'Dwyer ST, Smith RJ et al. Preservation
of small bowel mucosa using glutamine-enriched parenteral nutrition.
Surg Forum 1986; 37:56-58.
- Klimberg VS, Souba WW, Sitren H et al. Glutamine
enriched total parenteral nutrition supports gut metabolism. Surg
Forum 1989; 40:175-177.
- Ziegler TR, Benfell K, Smith RJ et al. Safety and
metabolic effects of L-glutamine administration in humans. J Parenter
Enteral Nutr 1990;14:137S-146S.
- Tamada H, Nezu R, Imamura I et al. The dipeptide
alanylglutamine prevents intestinal mucosal atrophy in parenterally
fed rats. J Parenter Enteral Nutr 1992; 16:119-116.
- Fox AD, Kripke SA, Paula JD et al. Effect of a
glutamine-supplemented enteral diet on methotrexate induced enterocolitis.
J Parenter Enteral Nutr 1988; 12:325-331.
- Klimberg VS, Souba WW, Dolsom DJ et al. Oral glutamine
supports crypt cell turnover and accelerates intestinal healing
following abdominal radiation. J Parenter Enteral Nutr 1989; 13:35.
- Souba WW, Klimberg VS, Hautamaki RD et al. Oral
glutamine reduces bacterial translocation following abdominal radiation.
J Surg Res 1990; 48:1-5.
- Souba WW. Klimberg VS. Copeland EM. Glutamine nutrition
in the management of radiation enteritis. J Parenter Enteral Nutr
1990; 14:106S-108S.
- Klimberg VS, Salloum RM, Kasper M et al. Oral glutamine
accelerates healing of the small intestine and improves outcome
after whole abdominal radiation. Arch Surg 1990; 125:1040-1045.
- Pegg AE. Recent advances- in the biochemistry of
polyamines in eukaryotes. Biochem J 1986; 234:249 262.
- Russell DH, Durie BGM. Ornihine decarboxylase -
a key enzyme in growth. Proc Congr Res Ther. 1978; 8:43-58.
- Luk GD, Marton LJ, Baylin SB. Ornithine decarboxylase
is important in intestinal mucosal maturation and recovery from
injury in rats. Science 1980; 210:195-198.
- Wang J, Johnson LR. Polyamines and ornithine decarboxylase
during repair of duodenal mucosa after stress in rats. Gastroenterology
1991; 100:333-343.
- Smith RJ. Panico KA. Automated analysis of Ophthalaldehyde
derivatives of amino acids in physiological fluids by reverse-phase
high performance liquid chromatography. J Liq Chromatogr 1985; 1783-1795.
- Bontemps J, Laschet J, Dnadrifosse G et al. Analysis
of dansyl derivatives of di-and polyamines in mouse brain, human
serum and duodenal biopsy by high-performance liquid chromatography
on a standard reversed-phase column. J chromatogr 1984; 311:59 67.
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurements
with Folin pheneal reagent. J Biol Chem 1951; 193:265-275.
- Presad AS, DuMouchelle E, Koniuch D et al. A simple
fluorometric method for the determination of RNA and DNA in tissues.
J Lab Clin Med. 1972; 80:598-602.
- Pinkus LM, Windmueller HG. Phosphate-dependent
glutaminase of small intestine: localization and role in intestinal
metabolism. Arch Biochem and Biophys. 1977; 182:506-517.
- Wright NA, Appleton DR. The metaphase arrest technique.
A critical review. Cell Tissue Kinet. 1980; 13:643-663.
- Bnarnason I, Smethurst PP, Leve AJ et al. Intestinal
permeability to 51Cr-EDTA in rats with experimentally
induced enteropathy. Gut 1985; 26:579 585.
- Jain R. Eikenburg BE, Johnson LR. Stimulation of
ornithine decarboxylase activity in digestive mucosa. Am J Physiol.
1987; 253:G303-G307.
- Minami H, Miyamoto K, Fujii Y et al. Induction
of intestinal ornithine decarboxylase by single amino acid feeding.
J Biochem. 1985; 133-139.
- Geraci JP, Jachson KL. Mariano MS. The intestinal
radiation syndrome: Sepsis and endotoxin. Radiation research 1985;
101:442-450.
- Bjarnason I. O'Morain C, Jonathan A, et al. Absorption
of 5'Cr-EDTA in inflammatory bowel disease. Gastroenterology 1983;
85:318-322.

Copyright © 1992 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
 to the top
to the top