Asia Pacific J Clin Nutr (l992) 1, 67-72
Serum bile acid fractions in neonates
on total parenteral nutrition is lithocholic acid responsible for
the occurrence of cholestasis?
Akio Kubota, Kenji Imura, Akira Okada*,
Shinkichi Kamata*, Riichiro Nezu* and Hisayoshi Kawahara*.
Department of Pediatric Surgery, Osaka
Medical Center and Research Institute for Maternal and Child Health,
Osaka, Japan and *Department of Pediatric Surgery, Osaka University
Medical School, Osaka, Japan.
In order to determine whether lithocholic acid (LCA) contributes
to the occurrence of total parenteral nutrition (TPN) associated
intrahepatic cholestasis (IHC) in neonates, we investigated the
serum bile acid fractions of neonates on TPN. Twenty-five surgical
neonates, receiving TPN for more than 2 weeks were studied. TPN
associated IHC was defined as serum defect bilirubin greater than
2.0 mg/dL. Serum bile acid fractions were examined by HPLC using
3a -hydroxy steroid dehydrogenase. Eight patients (32%;
IHC group) developed TPN associated IHC. Serum direct bilirubin
concentrations in the non IHC and IHC groups were 0.99 and 3.31
mg/dL respectively. Serum total bile acid levels in both groups
were 14.4 and 71.6 mmol/ml respectively. Glycine and taurine conjugated
cholic and chenodeoxycholic acids could be detected, and unconjugated
and secondary (deoxycholic and lithocholic) bile acid were detected
in trace levels in both the IHC and non-IHC groups. In conclusion,
LCA is unlikely to be a causative factor in TPN associated IHC in
neonates.
Introduction
Intrahepatic cholestasis especially in neonates frequently develops
during the course of total parenteral nutrition. Despite the vast
number of investigations dealing with its aetiology, the cause of
this TPN associated liver dysfunction remains unclear. This is now
considered to be related to various factors, including immaturity,
early fasting, surgical operations, underlying diseases, overloading
or imbalance of macronutrients, deficiency of trace elements, and
infection1,2,3. Our previous study revealed that in addition
to energy overloading, coexistence of infection and intestinal stasis
play major roles in IHC in neonates4.
Capron et al assumed that intestinal anaerobic bacterial overgrowth
could be a significant contributing factor to the occurrence of IHC
associated with TPN, and they showed that metronidazole, a drug which
suppresses anaerobic intestinal organisms, prevented the occurrence
of liver dysfunction during TPN in patients with chronic inflammatory
bowel disease (CIBD)5,6. We also demonstrated a beneficial
effect of metronidazole on TPN-associated liver dysfunction in surgical
neonates7. The results of these studies suggest that intestinal
overgrowth of anaerobic bacteria implicated in the occurrence of hepatic
dysfunction associated with TPN via certain mediators. Fouin-Fonunet
et al., in 1982, suggested a role of lithocholic acid (LCA)
in the IHC associated with TPN in patients with CIBD8.
In this study, in order to determine whether LCA contributes to the
occurrence of TPN-associated IHC in neonates, we investigated the
serum bile acid fractions.
Subjects
and methods
Twenty-five surgical neonates receiving TPN for more than 2 weeks
at our institutions between 1984 and 1987 were studied. Their age
ranged from 14 to 24 days, with a mean age of 17.4± 2.6 days. Their underlying diseases are shown in Table
1. The nutritional regimen contained 21% glucose, 2.5% amino acid,
and electrolytes, vitamins and trace elements. The solution was delivered
through a catheter placed in a central vein, and provided 7-100 kcal/kg/day
continuously. The amino acid mixture consisted of a formula devised
for paediatric use9. Blood samples for bile acid analysis
were collected in the morning under complete fasting with continuous
TPN infusion. Serum was collected by centrifugation immediately after
sampling of venous blood, and was stored at -80° C. The serum samples
were deproteinised and desalted with SEP-PAK C1810, and
fractionated into three groups using lipophilic gel chromatography11.
Each fraction was then applied to a high-performance liquid chromatography
system using an ODS column, and the fractionated cholic (CA), chenodeoxycholic
(CDCA), deoxycholic (DCA) and lithocholic acids (LCA) were applied
to the 3a -hydroxy steroid dehydrogenase-immobilised
column12. NADH that was generated in proportion to 3a
-hydroxy bile acids was measured by fluorometer (Figure 1). The external
standard of unconjugated, glycine conjugated and taurine conjugated
CA, CDCA, DCA and LCA were purchased from P-L Biochemicals, Inc, Milwaukee,
Wis. In this system using 3a -HSD, sulfated and glucuronidated bile acids were not detected,
since sulphate and glucuronide are conjugated with bile acid at the
position of 3a -OH.
All results are expressed as mean± SD.
Statistical analysis was performed by Student's t-test, and a probability
of 5% or less was considered significant.
Table 1. Underlying diseases.
| Oesophageal atresia |
6
|
| Duodenal atresia |
5
|
| Diaphragmatic hernia |
5
|
| Omphal ocele/gastroschisis |
3
|
| Midgut volvulus |
3
|
| Jejunal/Ileal atresia |
2
|
| Hirschsprung's disease |
2
|
| Necrotizing enterocolitis |
1
|
| CIIPS*1 |
1
|
| Total |
25
|
*1CIIPS: chronic idiopathic intestinal
pseudo-obstruction syndrome.
Figure 1. Procedure of serum bile acid analysis.-IHPLC: high-performance
liquid chromatography *23a -HSD: 3a -hydroxy steroid dehydrogenase
*3NAD: nicotinamide adeninedinucleotide. *4NADH:
dihydro-NAD.
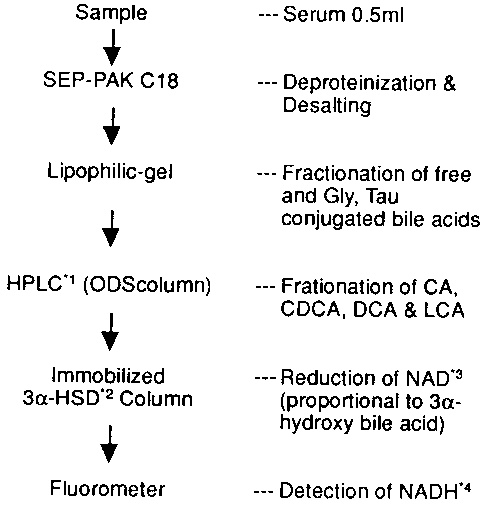
Table 2. Liver function tests.
| |
DB*1
(mg/dl) |
TBA*2
(nmol/ml) |
GOT*3
(iu/l) |
GPT*4
(iu/l) |
g -GTP*5
(iu/l) |
AL-P*6
(iu/l) |
| non-IHC*7 |
0.99± 0.59 |
14.4± 4.5 |
36.0± 55.0 |
14.6± 19.4 |
95.0± 66.1 |
302.4± 105.7 |
| IHC |
3.60± 1.69 |
71.7± 34.9 |
107.6± 166.6 |
23.8± 12.1 |
108.3± 77.4 |
239.1± 105.7 |
| P values |
P<0.01 |
P<0.01 |
NS |
NS |
NS |
NS |
*1DB: direct bilirubin. *2TBA: total bile acid.
*3GOT: glutamic ozaloacetic tranaminase. *4GPT:
glutamic-pyruvic transaminase. *5g
-GTP: g -glutamyl transpeptidase.
*6AL-P: alkaline phoshatase. *7IHC: intrahepatic
cholestasis
Results
Liver
function tests
Table 2 shows the results of routine liver function tests. Out of
25 patients, 8 (32%) developed cholestasis with a serum concentration
of direct bilirubin greater than 2.0 mg/dl during the first month
of life. These 8 patients were included in the IHC group, and
the other 17 in the non-IHC group. The mean serum concentrations of
direct bilirubin in the non-IHC and IHC groups were 0.99 and 2.60
mg/dl, respectively, and those of total bile acid were 14.4 and 71.7
nmol/ml, respectively. Thus, total bile acid concentration was significantly
higher in the IHC group. There was no significant difference in glutamic
oxaloacetic transaminase, glutamicpyruvic transaminase, g -glutamyl transpeptidase and alkaline phosphatase between
the non-IHC and IHC groups.
Serum
bile acid fractions
In the non-IHC group, the mean glycine- and taurine conjugated cholic
acid were 4.9 and 3.1 nmol/ml, respectively (Table 3). Glycine- and
taurine-conjugated chenodeoxycholic acids were 3.1 and 2.3 nmol/ml,
respectively. However unconjugated bile acids or secondary bile acids,
such as deoxycholic or lithocholic acid were not detected or were
detected only in trace levels. In the IHC group, these fractions were
detected in high amounts; glycine-conjugated bile acids were approximately
four times higher than in the non-IHC group, and taurine-conjugated
bile acids were approximately seven times higher than in the non-IHC
group. However, in the IHC group, unconjugated and secondary bile
acids were not detected or were detected only in trace levels. There
was no marked increase in any single fraction in the IHC group.
Discussion
Certain bile acids, in particular the monohydroxylated secondary
bile acid, LCA, are known to be cholestatic in a variety of animal
species13-19. The histologic changes produced in LCA-treated
animals13,15,16 are similar to the changes observed in
neonates and infants with TPN-associated IHC4,20-24. This
suggests that the histologic changes seen in patients receiving TPN
could be attributed to LCA. Intrinsic LCA is normally produced in
the small intestine and colon by anaerobic bacterial dehydroxylation
of chenodeoxycholic acid25. Recently, several investigators
have reported the beneficial effects of metronidazole, a drug which
suppresses intestinal anaerobic organisms26, on TPN-associated
liver dysfunction in adult patients with CIBD5,6, in surgical
neonates7, and in animals27. These reports suggest
that hepatotoxic substances produced by anaerobic intestinal bacteria
contribute to the occurrence of liver dysfunction during TPN. Fouin-Fortunet
et al. showed that in TPN patients with CIBD, the biliary concentration
of LCA was significantly higher in patients with hepatic dysfunction
than in patients with normal liver function8. Balistreri
et al. found increased serum levels of sulfated LCA in infants
receiving TPN who developed IHC28,29. Above studies suggest
that intrinsic LCA may also cause liver dysfunction. However, no reports
have yet shown that intrinsic LCA causes TPN-associated liver
dysfunction in infants. We therefore investigated the serum bile acid
fraction in neonates on TPN in this study, and attempted to determine
whether LCA contributes to the occurrence of TPN-associated liver
dysfunction in neonates. Sulfate and glucuronide conjugated bile acid
were not analysed; however, Stiehl demonstrated that approximately
20% of serum LCA in infants with cholestasis was non-sulphated and
non-glucuronated LCA30, indicating that if sulphated or
glucuronated LCA is increased, nonsulphated or glucuronated
LCA is also increased. The present study showed that no single fraction,
including LCA, was increased, even in patients with IHC, suggesting
that LCA is unlikely to be a causative factor in TPN-associated
IHC in neonates. In the previous studies reported by Stiehl or Farrell
et al., serum LCA concentrations were as high as 2-4 m
g/ml (approximately 4-8 mmol/ml). As reported by Stiehl et al.,
these concentrations were much lower than the amount given to animals
to induce cholestasis, 120-240 mol/kg intravenous infusion14,17,18,19and
0.1-1% in oral feedingl3,15. And he concluded that it unlikely
that such concentrations of monohydroxy bile acids measured in their
patients were responsible for the cholestasis30. Furthermore,
Cano et al. reported that in 8 non-CIBD patients with TPN-induced
cholestasis, biliary LCA was normal; and in 6 cases with normal enterohepatic
cycle where bile acid composition was normal, LCA represented less
than 1% of total bile acids31. They concluded that LCA
could not account for the occurrence of cholestasis in their patients.
Table 3. Serum bile acid fractions.
| Fraction |
non-IHC group
|
IHC-group
|
P values
|
| G*1 -CA*7 |
4.9± 2.5
|
22.8± 16.6
|
<0.05
|
| T*2 -CA |
3.1± 2.0
|
22.6± 12.2
|
<0.05
|
| U*3 -CA |
trace
|
trace
|
-
|
| G-CDCA*8 |
3.1± 1.1
|
12.7± 7.7
|
<0.05
|
| T-CDCA |
2.3± 1.2
|
16.6± 10.2
|
<0.05
|
| U-CDCA |
trace
|
trace
|
-
|
| G- DCA*9 |
trace
|
trace
|
-
|
| T- DCA |
trace
|
trace
|
-
|
| U- DCA |
trace
|
trace
|
-
|
| G- LCA*10 |
trace
|
trace
|
-
|
| T- LCA |
trace
|
trace
|
-
|
| U- LCA |
trace
|
trace
|
-
|
| Total CA |
8.5± 4.1
|
46.3± 23.2
|
<0.05
|
| Total CDCA |
5.4± 1.6
|
24.7± 16.6
|
<0.05
|
| Total DCA |
trace
|
trace
|
-
|
| Total LCA |
trace
|
trace
|
-
|
| Total Cly*4 |
8.0± 3.1
|
35.5± 23.4
|
<0.05
|
| Total Tau*5 |
5.9± 3.0
|
39.5± 20.4
|
<0.05
|
| Total Unc*6 |
trace
|
trace
|
-
|
| TBA |
14.4± 4.5
|
76.1± 34.4
|
<0.05
|
*1G: Glycine conjugated. *2: taurine conjugated.
*3: Unconjugated *4Gly: Glycine conjugated bile
acids. *5:Taurine conjugated bile acid. *6Unc:
Unconjugated bile acids. *7CA: cholic acid. *8CDCA:
Chenodeoxycholic acid. *9DCA: deoxycholic acid. *10DCA:
lithocholic acid.
Figure 2. Mechanisms of TPN-associated intrahepatic cholestasis.
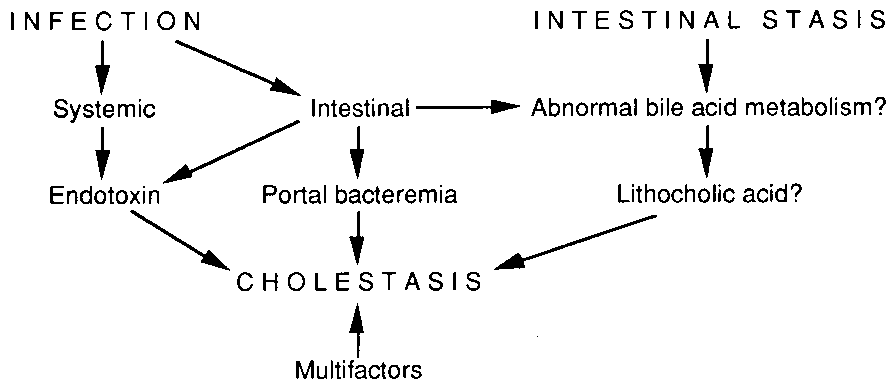
The results of our previous study showing that coexistence of infection
and intestinal stasis were two major contributing factors in the occurrence
of IHC during TPN in neonates4, and the beneficial effects of metronidazole
on this TPN-associated liver dysfunction567'7 indicate the role of
another sepsis-mediated mechanism such as endotoxin release or portal
bacteremia (Figure 2). Cholestatic effects of endotoxin have been
demonstrated in isolated perfused rat liver by Utili et al.32.
Because young infants are highly susceptible to endotoxin, it is reasonable
to presume that endotoxin may induce liver dysfunction in neonates
on TPN. The observation of pericholangitis in cases of TPN-associated
liver dysfunction4,21,22, supports the possibility that
ascending cholangitis is caused by portal bacteremia secondary to
intestinal bacterial overgrowth, and results in intrahepatic cholestasis.
Thus, portal bacteremia is another possible route for sepsis-mediated
mechanism. Further investigations are necessary to clarify the relationship
between intestinal or systemic bacterial proliferation and TPN-associated
liver dysfunction in infants.
Correspondence:
Akio Kubota, 840 Murodo-cho, Izumi, Osaka 590 02, Japan.
References
- Merritt RJ. Cholestasis associated with total parenteral nutrition.
I Pediatr Gastroenter Nutr 1986; 5:9-22.
- Bell RL, Ferry GD, Smith EO, et al. Total parenteral nutrition-related
cholestasis in infants. JPEN 1986; 10:356-359.
- Drongowski RA, Coran AG. An analysis of factors contributing to
the development of total parenteral nutrition-induced cholestasis.
JPEN 1989; 13: 586-589.
- Kubota A, Olcada A, Nezu R, et al. Hyperbilirubinemia in
neonates associated with total parenteral nutrition. JPEN 1988;
12: 602-606.
- Capron JP, Gineston JL, Herve MA, et al. Metronidazole
in prevention of cholestasis associated with total parenteral nutrition.
Lancet 1983; 1: 446-447.
- Lambert JR, Thomas SM. Metronidazole prevention of serum liver
enzyme abnormalities during total parenteral nutrition. 1985; JPEN
9: 501-503.
- Kubota A, Okada A, Imura K, et al. The effect to metronidazole
on TPN-associated liver dysfunction in neonates. J Pediatr Surg
1990; 25: 618-621.
- Fouin-Foutunet H, LeOuernec L, Erlinger S, et al. Hepatic
alterations during total parenteral nutrition in patients with inflammatory
bowel disease: possible consequence of lithocholic toxicity. Gastroenterol
1982; 82: 932-937.
- Imura K, Fukui Y, Kawahara H, et al. Clinical studies on
a newly devised amino acid solution for neonates. JPEN 12: 1988;
496-504.
- Goto J, Kato H, Saruta Y, et al. Separation and determination
of bile acids in human bile by high-performance liquid chromatography.
J Liquid Chromatogr 1980; 3: 991-1003.
- Goto J, Hasaegawa M Kato H, et al. A new method for simultaneous
determination of bile acids in human bile without hydrolysis. Clinical
Chimica Acta 1978; 87: 141-147.
- Kamada S, Maeda M, Tsuji A. Separation and determination of bile
acid by high-performance liquid chromatography using immobilized
3a -hydroxy steriod dehydrogenase and
an electrochemical detector. J Chromatogram 1982; 239: 773-783.
- Hunt RD, Leveille GA, Sauberlich HE. Dietary bile acids and lipid
metabolis. III. Effects of lithocholic acid in mammalian species.
Proc Soc Exp Biol Med 1964; 115: 277-280.
- Javitt NB. Cholestasis in rats induced by taurolithocholate. Nature
1966; 210: 1262-1263.
- Palmer RH, Hruban Z. Production of bile duct hyperplasia and gallstone
by lithocholic acid. J Clin Invest 1966; 45: 1255-1267.
- Fischer CD, Cooper NS, Rothschild MA, et al. Effect of
dietary chenodeoxycholic acid and lithocholic acid in the rabbit.
Am J Dig Dis 1974; 19: 877-886.
- Layden TJ, Boyer JL. Taurolithocholate-induced cholestasis: taurocholate,
but not dehydrocholate, reverses cholestasis and bile canalicular
membrane injury. 1977; 73: 120-128.
- Kakis G, Yousef IM. Pathogenesis of lithocholate- and taurocholate-induced
intrahepatic cholestasis in rats. Gastroenterol 1978; 75: 595-607.
- Yousef IM, Tuchweber B, Vonk RJ, et al. Lithocholate cholestasis-sulfated
glycolithocholate-induced intrahepatic cholestasis in rats. Gastroenterol
1981, 80: 233-241.
- Cohen C, Olsen MM. Pediatric total parenteral nutrition: liver
histopathology. Arch Patrol Lab Med 1980; 105: 152-156.
- Dahms BB, Halpin TC Jr. Serial liver biopsies in parenteral nutrition-associated
cholestasis of early infancy. Gastroenterol 1981; 81: 136-144.
- Benjamin DR. Hepatobiliary dysfunction in infants and children
associated with long-term total parenteral nutrition. A clinical-pathologic
study. Am J Clin Pathol 1981; 76: 276-283.
- Hodes JE, Grosfeld JL, Weber TR, et al. Hepatic failure
in infants on total parenteral nutrition (TPN): Clinical and histopathologic
observations. J Pediatr Surg 1982; 17: 463-468.
- Hughes CA, Talbot IC, Ducker DA, et al. Total parenteral
nutrition in infancy: effect on the liver and suggested pathogenesis.
Gut 1983; 24: 241-248.
- Macdonald IA, Bokkenheuser VD, Winter J, et al. Degradation
of steroids in the human gut. J Lipid Res 1983; 24: 675-700.
- Goldman P. Metronidazole. N Engl J Med 1980; 20: 1212-1218.
- Freund HR, Muggia-Sullam M, LaFrance R, et al. A possible
beneficial effect of metronidazole in reducing TPN-associated liver
function derangements. J Surg Res 1985; 38: 356-363.
- Balistreri WF, Suchy FJ, Farell MK, et al. Pathologic versus
physiologic cholestasis: elevated serum concentration of a secondary
bile acid in the presence of hepatobiliary disease. J Pediatr 1981;
98: 399-402.
- Farell MK, Balistreri WF, Suchy FJ: Serum-sulfated lithocholate
as an indicator of cholestasis during parenteral nutrition in infants
and children. JPEN 1982; 3: 30-33.
- Stiehl A, Becker M, Czygan P, et al. Bile acids and their
sulphated and glucuronidated derivatives in bile, plasma, and urine
of children with intrahepatic cholestasis: effects of phenobarbital
treatment. Eur J Clin Invest 1980; 10: 307-316.
- Cano N, Gerolami A. Intrahepatic cholestasis during total parenteral
nutrition. Lancet 1983; 1 (8331): 985.
- Utili R, Abernathu CO, Zimmermann HJ. Cholestatic effects of escherichia
coli endotoxin on the isolated perfused rat liver. Gastroenterol
1976; 70: 248-253.

Copyright © 1996 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 14, 1999
.
to the top