Asia Pacific J Clin Nutr (1993) 2, Suppl 1, 21-25
Vitamins A, C, E and b-carotene as protective factors for some cancers
Ivor
E. Dreosti, PhD, DSc
CSIRO Division of Human Nutrition, Adelaide,
SA, Australia.
The importance of the antioxidant micronutrients
vitamins A, C, E and b -carotene in cancer prevention is currently
a widely debated human health issue. Generally supported by laboratory
findings, and persuasively linked to the lower cancer risk associated
with high intakes of fruit and vegetables, the hypothesis is now
being tested in many prospective studies around the world. Increasingly,
oxidative damage has been implicated in the etiology of several
degenerative diseases including cancer, thus highlighting the need
to ensure replete antioxidant nutriture as a central measure in
preventive medicine.
Introduction
Environmental factors are considered to be responsible
for the development of 80-90% of cancers in humans, of which 30-60%
exhibit strong dietary links. Overall it has been estimated that appropriate
dietary alterations could prevent about 1/3 of cancer eases in humans1.
Such strategies would include on the one hand the avoidance of carcinogenic
substances in food, while on the other attempting to increase the
intake of dietary anticarcinogens.
Dietary anticarcinogens
Many substances in food have been nominated as candidate
anti-carcinogens, ranging from macronutrients such as fibre
and calcium to a large number of minor dietary constituents including
several micronutrients. The mechanisms whereby these substances may
act protectively are widely diverse and are summarized in Table 1.
Table 1. Mechanisms of cancer protection by
minor dietary constituents*.
- Prevent carcinogen formation
- Block carcinogen action
- reduce metabolic activation
- increase detoxification (cytochrome P-450,
conjugation)
- Reduce free radical related cellular damage
- antioxidants
- Enhance error-free DNA repair
- Suppress cancer expression (reversible)
- oncogene control
- cellular differentiation
- Enhance immunosurveillance
|
* Developed from references2-5
Pro-oxidants,
antioxidants and carcinogenesis
Of particular current interest are the antioxidant
micronutrients which include vitamins A, C, E and the carotenoids
and which are considered to act protectively in several ways including
limiting oxidative free radical damage to DNA and other cellular macromolecules
(Figure 1), (Table 2).
Figure 1. Cellular free radical-related damage
and antioxidant micronutrient defence.
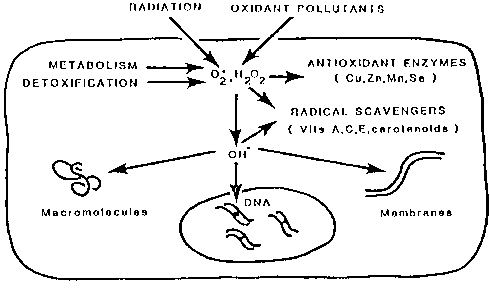
Table 2. Possible mechanisms of vitamins A,
C, E and , b -carotene vitamins in cancer protection*.
| Mechanism |
Active Agent |
| 1 Reduced carcinogen
formation |
Vitamins C, E |
| 2 Reduced oxidative
damage |
Vitamins A, C, E, b -Carotene |
| 3 Reduced oncogene expression
(myc, ras) |
Vitamins A, E |
| 4 Reduced cell signalling
systems (AC, PKC) |
Vitamins A, E, b - Carotene |
| 5 Induced differentiation
of transformed cells |
Vitamins A, C, E, b -Carotene |
| 6 Enhanced immunocompetence
|
Vitamins A, C, E, b -Carotene |
*Developed from references1-4,6-8.
Certainly, many pro-oxidants are potent experimental
carcinogens (Table 3), while many antioxidants have been demonstrated
to be significantly protective against cancer.
Table 3. Pro-oxidants as carcinogens7.
| Radiation (X and UV)
Hyperbaric oxygen
Peroxides
Peroxisome proliferators
clofibrate
nafenopin
Modulators of electron transport chain
rotenone
phenobarbital
|
Xenobiotic metabolism
daunorubicin
streptonigrin
adriamycin
mitomycin C
polycyclic aromatic hydrocarbons
Antioxidant inhibitors
phorbol myristate acetate
Asbestos
|
Evidence for the role of antioxidants as antimutagens/
anticarcinogens has emerged from three areas of study.
- In vitro experiments with cells in culture exposed
to pro-oxidant mutagens/carcinogens and various antioxidants.
- In vivo studies with experimental animals following
a similar experimental protocol.
- Human epidemiological surveys.
Both the experimental animal studies and the human
surveys are especially relevant to the assessment of the antioxidant
vitamins as important dietary anticarcinogens.
Vitamins
A, C, E and carotene: animal studies
Several hundred experimental studies have addressed
the issue of the antioxidant vitamins and b -carotene as anticarcinogens with
respect to a variety of potent carcinogens in several animal species
- often with equivocal results (Table 4).
Table 4. Vitamins A, C, E and b -carotene as anticarcinogens -
animal studies* .
| Nutrient |
Species |
Organ |
Carcinogen** |
Overall effect on carcinogenesis |
| Vitamm A |
rat |
colon |
NG |
if deficient |
| (Retinoids) |
rat |
lung |
MCA |
if deficient |
| |
hamster |
lung |
BP/MCA |
¯ ® |
| |
mouse |
skin |
BP/DMBA-TPA |
¯ |
| |
rat |
mammary gland |
DMBA/NU/virus |
¯ |
| |
mouse |
mammary gland |
virus |
¯ |
| |
rat |
bladder |
NU/NA |
¯ ® |
| |
mouse |
bladder |
NH |
¯ |
| Vitamin C |
mouse |
skin |
UV/DMBA-CO |
¯ |
| |
hamster |
naso-trachea |
Cig-Smoke/NA |
¯ / |
| |
mouse |
lung |
NA |
¯ |
| |
mouse |
lung |
fibreglass |
¯ |
| |
hamster |
kidney |
estrogen |
¯ |
| |
rat |
colon |
DMH |
¯ ® |
| |
mouse |
colon |
DMH |
® |
| |
rat |
mammary gland |
DMBA |
® |
| |
rat |
bladder |
NA |
® |
| |
mouse |
bladder |
NA |
¯ |
| |
rat |
sarcoma |
BP |
¯ |
| Vitamin E |
mouse |
skin |
DMBA-CO/UV |
¯ / ¯ |
| |
hamster |
mouth |
DMBA |
¯ |
| |
rat |
mammary gland |
DMBA/DN/NU |
¯ ® |
| |
mouse |
forestomach |
DMBA |
® |
| |
rat |
colon |
DMH/DMBA |
¯ ® / |
| |
mouse |
colon |
DMH |
¯ / |
| |
rat |
liver |
DMAB/NA |
¯ |
| |
hamster |
liver |
NA |
¯ |
| b -Carotene |
mouse |
skin |
UV |
¯ |
| |
rat |
salivary gland |
DMBA |
¯ |
| |
hamster |
mouth |
DMBA |
¯ |
| |
mouse |
mammary gland |
MOP |
¯ |
| |
rat |
stomach |
NG |
¯ ® |
| |
mouse |
colon |
DMH |
¯ |
| |
mouse |
sarcoma |
virus |
¯ |
*Compiled from references2.,6,9-11.
BP - benzo (a) pyrene; CO - croton oil; DMAB-dimethylazobenzene;
DMH - dimethylhydrazine; DN - daunomycin; MCA - methylcholamthrene;
MOP - methoxypsoralen; NA - nirosamine; NG-nitrosoguanidine; NU -
nitrosourea; TPA - phorbol acetate
increase; à no effect; ¯ decrease
Overall however, the weight of evidence points persuasively
to protection by each of the agents with respect to skin carcinogenesis,
retinoids in relation to the mammary gland, vitamin C with respect
to the lung, vitamin E for tumours of the mouth and liver, while b -carotene appears to be generally protective for the mouth, mammary
gland and colon.
Vitamins
A, C, E and carotene: human studies
To date, most human studies concerning the anticancer
activity of vitamins A, C, E and the carotenoids have been of a non-experimental
epidemiological nature, which, while providing valuable leads to the
underlying science, cannot because of methodological limitations,
draw precise conclusions with respect to single nutrients (Table 5).
Table 5. Categories of human diet-cancer studies*.
| Epidemiological studies (non-experimental)
Dietary-intake
- Food disappearance data - good for international
trends but many confounders/associations.
- Cohort studies (prospective) - excludes cancer
as a confounder but associations still present.
- Case control studies (retrospective) - fewer
confounders but dietary history data imprecise.
Nutrient Status
- Tissue analysis - suitable for case or cohort
studies but potential bias due to presence of cancer at the
time of sampling.
Intervention studies (experimental)
Preclinical Trials (3 stages)
- Selection of agent.
- Test effectiveness of agent.
- Establish pharmacology and toxicology.
Clinical Trials (3 phases)
- Establish dose and safety in humans (chemoprevention
vs treatment).
- Establish effectiveness of agent in humans.
- Conduct human trials for risk reduction (prospective,
at risk populations).
|
*Compiled from references4-6,12,13.
Nevertheless, the very large number of studies performed
so far point overwhelmingly to protection against many forms of cancer
by high intakes of fruit and vegetables (Table 6), with strong
associations emerging in some cases between the estimated dietary
intake or monitored nutrient status of the particular micronutrients.
Table 6. A, C and E vitamins and b -carotene as anticarcinogens -
human studies*.
| Agent |
Target organ |
Overall cancer risk |
| Fruit & Vegetables |
all sites |
¯ |
| |
mouth, larynx, esophagus, |
¯ |
| |
stomach colorectum, |
¯ |
| |
pancreas, lung, bladder, |
¯ |
| |
cervix, endometrium |
¯ |
| Vitamin A (Retinoids) |
skin |
¯ |
| |
mouth (leukoplakia) |
¯ |
| |
esophagus, bladder, |
¯ |
| |
lung |
¯ à |
| Vitamin C |
mouth |
¯ à |
| |
larynx, esophagus, stomach |
¯ |
| |
colorectum, |
¯ à |
| |
pancreas, cervix, |
¯ |
| |
lung |
¯ à |
| Vitamin E |
all sites |
¯ à |
| |
esophagus, stomach |
¯ à |
| |
intestine |
à |
| |
breast |
¯ à (¯ selenium aggravates) |
| |
pancreas, bladder |
¯ à |
| b -carotene |
skin (melanoma) |
à |
| |
mouth |
¯ à |
| |
lung, esophagus, stomach |
¯ |
| |
pancreas, bladder, colorectum |
¯ à |
| |
breast, cervix, prostate |
¯ à |
*Compiled from references1,5,6,9,11,13-20
and include epidemiological studies of all types, and assessment of
nutrient status by inference from dietary data and by analysis of
levels in blood serum of A, C and E vitamins and b -carotene.
In addition, some use has been made clinically, and
in an experimental setting, of vitamins A, C, E and carotenoids in
cancer therapy (Table 7), but detailed discussion of this topic is
outside the scope of this review.
Table 7. Use of A, C and E vitamins and b -carotene in cancer therapy*.
| Agent |
Condition |
Vitamin A
(Retinoids) |
actinic keratosis, keratoacanthoma
oral leukoplakia
lung metaplasia
post surgery ± chemo/radiotherapy |
| Vitamin C |
- |
| Vitamin E |
- |
| b -Carotene |
oral leukoplakia
post surgery ± chemo/radiotherapy |
*Compiled from references5,14,21,22.
Clearly, very much more definitive data will become
available over the next decade from the many human intervention trials
which are now under way around the world. Table 8 summarizes some
of the major intervention programs being conducted by the USA National
Cancer Institute, and some by Australian workers. The magnitude of
resources committed to these studies bears witness to the strong belief
among nutritional scientists that dietary antioxidants may indeed
offer a valuable tool in cancer prophylaxis.
Table 8. Some current chemoprevention trials
with A,C or E vitamins or b -carotene in humans*.
| Agent |
Target organs |
Risk group |
No. of studies |
Location |
| Vitamin A |
all sites |
dental nurses |
1 |
USA |
| (Retinoids) |
skin |
keratoses, BCC** |
3 |
USA |
| |
lung |
smokers |
4 |
Australia, USA |
| |
cervix |
dysplasia |
1 |
USA |
| |
colon |
polyposis |
1 |
USA |
| |
colon/rectum |
polyposis |
1 |
USA |
| Vitamin C |
skin |
BCC |
1 |
USA |
| |
colon |
adenomas, polyposis, normal |
3 |
USA |
| Vitamin E |
all sites |
dental nurses |
1 |
USA |
| |
skin |
BCC |
1 |
USA |
| |
lung |
smokers |
2 |
USA |
| |
colon |
polyposis, normal |
3 |
USA |
| |
colon/ rectum |
polyposis |
1 |
USA |
| b -Carotene |
all sites |
skin lung |
1 |
USA |
| |
skin |
BCC, albinos |
3 |
Australia, Tanzania, USA |
| |
lung |
asbestos(is) smokers, aged |
5 |
Australia, Finland, USA |
| |
esophagus |
dysplasia |
2 |
China, USA |
| |
colon |
adenomas, polyposis |
3 |
Australia, USA |
| |
cervix |
dysplasia |
2 |
Australia, USA |
| Multivitamins |
all sites |
- |
1 |
USA |
| |
esophagus |
high risk areas, general |
1 |
China, USA |
*Compiled from references12,23.
**Basal cell carcinoma.
Interpreting
the present dilemma
In the mean time, before data from the definitive
intervention trials is forthcoming what broad conclusions may be drawn
from existing findings? Clearly, the evidence for protection by high
intakes of fruit and vegetables is impressive and argues strongly
for continuing nutritional education along these lines, since these
foodstuffs contain many other putative protective factors as minor
dietary constituents apart from the antioxidants.
However, in terms of current recommended dietary intakes
(RDIs) it should be noted that the very high levels of consumption
of fruit and vegetables needed to confer protection against cancer,
when extrapolated to the putative micronutrients involved, reflects
intakes considerably in excess of current RDI's. Unquestionably, fruits
and vegetables are complex mixtures of many food factors and there
can be no firm assurance that the nominated antioxidant micronutrients
are the main active agents - except for the strong support from experimental
studies with animals, and several recent human surveys linking reduced
risk of cancer specifically to antioxidant supplementation (Table
9). There seems little doubt that the stage has now been reached when
nutritional scientists need to consider carefully the criteria on
which the requirements for some micronutrients are based. Prevention
of overt deficiency disease or apparently adequate reserves may not
establish the requirement for optimum health and protection against
degenerative disease. For example, in rats it appears that normal
growth can be obtained on a diet containing 7.5 mg vitamin E/kg. Myopathy
can be prevented with 15mg/kg and red cell haemolysis avoided with
50mg of vitamin E/kg.
Table 9. Evidence of cancer protection by A,
C and E vitamin supplementation* .
| Agent |
Target organ |
Risk:odds ratio |
Dose |
| Vitamin E |
mouth and pharynx |
0.5 |
usage of supplements |
| Vitamin C |
colon and rectum |
0.5 |
>230 mg/day |
| Vitamin C |
lung cancer (smokers) |
0.3 |
high in diet plus supplements |
*Compiled from references19,24,25.
However, the mitogenic response of T and B lymphocytes,
which reflects a measure of immunocompetence, increases linearly with
a rise in serum vitamin E levels between 0.4-18m
g/ml obtained from diets containing 200mg/kg26.
The possibility therefore arises that the evolution
of certain aspects of human micronutrient metabolism did not take
place in the context of present dietary intakes and lifestyle factors.
Paleolithic man for example is estimated to have consumed around 400mg
of vitamin C each day27. The diet scientists perceive today
to be well balanced may conceivably not be precisely the optimum for
maximum human health.
References
- Jensen J, Madsen JL. Diet and cancer. Review of
the literature. Acta Med Scand 1988; 223:293-304.
- Prasad KN, Edwards-Prasad J. Expression of some
molecular cancer risk factors and their modification by vitamins.
J Am Coll Nutr 1990; 9:28-34.
- Wattenberg LW. Inhibition of carcinogenesis by
minor dietary constituents. Cancer Res 1992; 52:2085s-91s.
- Birt DF, Bresnick E. Chemoprevention by non-nutrient
components of vegetables and fruits. In: Alfin-Slater RB, Kritchevsky
D, eds. Cancer and nutrition. New York: Plenum Press; 1991 :221-61.
- Merrill AH, Foltz AT, McCormick DB. Vitamins and
cancer. In: Alfin-Slater RB, Kritchevsky D, eds. Cancer and nutrition.
New York: Plenum Press; 1991:262-320.
- Dorgan JF, Schatzkin A. Antioxidant micronutrients
in cancer prevention. Hematol/Oncol Clinics N Am 1991; 5:43-68.
- Cerutti PA. Pro-oxidant states and tumor promotion.
Science 1985; 227:375-81.
- Verma AK. Modulation of carcinogenesis by vitamin
A and its analogs (retinoids). In: Laidlaw SA, Swendseid ME, eds.
Vitamins and cancer prevention. New York: Wiley-Liss; 1991:61-90.
- Carpenter MP. Vitamins E and C in neoplastic development.
In: Laidlaw SA, Swendseid ME, eds. Vitamins and cancer prevention.
New York: Wiley-Liss, 1991; 27-37.
- Moon R. Retinoids and prevention of experimental
cancer. In: Prasad KN, Meyskens Jr FL, eds. Nutrients and cancer
prevention. Clifton, New Jersey: Humana Press; 1990:197-206.
- Chen LH, Boissonneault GA, Glauert HP. Vitamin
C, vitamin E and cancer. Anticancer Res 1988; 8:739-48.
- Malone WF. Chemoprevention research. In: Weisburger
EK, ed. Mechanisms of carcinogenesis. Dordrecht: Kluwer Academic
Pubs; 1989:31-42.
- Steinmetz KA, Potter HD. Vegetables, fruit, and
cancer. 1. Epidemiology 2. Mechanisms. Cancer causes and control
1991; 2:325--57 and 427-42.
- Greenberg ER. An approach to studying the possible
effects of carotenoids in skin cancer prevention. In: Laidlaw SA,
Swenseid ME, eds. Vitamins and cancer prevention. New York: Wiley-Liss;
1991:1-14.
- Block G, Patterson B, Subar A. Fruit, vegetables
and cancer prevention: A review of the epidemiological evidence.
Nutr Cancer 1992; 8:1-29.
- Prasad MPR, Krishna TP, Pasricha S, Krishnaswamy
K, Quereshi MA. Esophageal cancer and diet - A case control study.
Nutr Cancer 1992; 18:85-92.
- Hartman PE, Shanker DM. Antimutagens and anticarcinogens.
A survey of putative interceptor molecules. Env Mol Mutagenesis
1990; 15:145-182.
- Rutishauser IHE. Vitamin A and the carotenoids
in human nutrition. In: Walker AF, Rolls BA, eds. Nutrition and
the consumer. London: Elsevier; 1992:17-68.
- Block G. Epidemiologic evidence regarding vitamin
C and cancer. Am J Clin Nutr 1991; 54:1310s-14s.
- Meyskens FJ Jr. Control of human preneoplasia with
retinoids and other compounds. In: Prasad KN, Keyskens FL Jn, eds.
Nutrients and cancer prevention. Clifton, New Jersey: Humana Press;
1990:289-298.
- Santamaria L, Santamaria AB, dell'Orti M. Carotenoids
in cancer chemoprevention and synergism with retinol in mastalgia
treatment. In: Prasad KN, Meyskens FL Jr, eds. Nutrients and cancer
prevention. Clifton, New Jersey: Humana Press; 1990:299-317.
- Stich HF, Mathew B, Sankaranarayanan R, Krishnan
Nair M. Vitamin A and b -carotene; long term protective
effects in oral leukoplakia. In: Laidlaw SA, Swendseid ME, eds.
Vitamins and cancer prevention. New York: Wiley-Liss; 1991:15-24.
- Pinnock CB, Mackerras D, Meyskens FL Jr. Dietary
anticancer agents: b -carotene, retinoids and beyond.
Report of a workshop held in Adelaide November 1990, in conjunction
with the Nutrition Society of Australia National Conference. 1991;
2-15.
- Kune S, Kune GA, Watson LF. Case-control study
of dietary etiological factors: The Melbourne colorectal cancer
study. Nutr Cancer 1987; 9:21-42.
- Gridley G, McLaughlin JK, Block G, Blot WJ, Cluch
M, Fraumeni JF Jr. Vitamin supplement use and reduced risks of oral
and pharyngeal cancer. Am J Epidemiol 1992; 135: 1083-92.
- Bendich A, Gabriel E, Machlin LJ. Dietary vitamin
E requirement for optimum immune responses in the rat. J Nutr 1986;
116:675-81.
- Eaton SB, Konner M. Paleolithic nutrition. New
Eng J Med 1985; 312:283-90.

Copyright © 1993 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
to the top