|
|
Asia Pacific J of Clin Nutr. (1995) 4, Suppl 1, 29-33
Asia Pacific J of Clin Nutr. (1995) 4, Suppl 1, 29-33

Benefits of physical activity on
nutrition and health status: studies in China
Chen Ji-Di, MD
Research Division of Sports Nutrition
and Biochemistry, Institute of Sports Medicine, Beijing Medical University,
Beijing, China.
The significance of physical activity for fitness
is that it may change risk factors for chronic disease and improve
functional and psychological status. Rational nutrition and scientific
training are prerequisites for safe and useful exercise. Even mild
iron deficiency anemia can affect physical capacity. Intensive exercise
has been found to increase serum and erythrocyte lipid peroxide
levels. Moderate exercise decreases the blood lipid peroxides and
increases free radical elimination enzyme (SOD, GSH-Px, CAT) activities.
Elite athletes have significantly lower Malonic dialdehyde (MDA)
levels and higher SOD and GSH-Px activities. Zinc deficiency not
only leads to an increase in free radical generation and lipid peroxidation
and a depression of SOD activity, but also is harmful to immune
function. Exercise can exacerbate the damage induced by iron deficiency
or zinc deficiency. Long term diet restriction by gymnasts to control
weight showed detrimental effects including growth retardation,
menstrual disturbances, malnutrition, mental stress and muscle weakness.
Comprehensive nutrition promotes growth rate and corrects malnutrition
without a body fat increase in athletes. Exercise benefits the growth
and development of bone and muscle and enhances muscle strength.
The prevention of obesity is particularly important during periods
of rapid growth. The establishment of an exercise life style during
childhood will favor the best health.
Introduction
The importance of exercise for fitness is becoming
clearer every day and it is a popular topic of conversation. People
generally have a surface knowledge of the following:
1. Exercise may lower some risk factors for chronic
diseases. Physical demands at work, at home and in transit have been
dramatically reduced by modernization. People are leading a more and
more sedentary lifestyle that leads to a progressive increase of such
chronic diseases as obesity, coronary heart disease, diabetes, osteoporosis,
and back pain. Exercise combined with a proper diet lowers certain
risk factors for chronic disease. Thus it has important implications
for health and is worth recommendation1-4.
2. Exercise improves human functional status and metabolism.
The oxygen uptake (V02), maximal oxygen uptake (V02
max), cardiac output (CO), and stroke volume increased significantly
after running training5. Heart rate recovered faster after
a 30 meter run at full speed in exercise trained children than non-trained
controls6. Microcirculation, heart function, and lipid
profiles improved in aged peopl 1000 e that jog or practice Qi-gong
or Tai-chi as compared to control subjects4,7-10.
3. Exercise improves psychological state and capacity
to cope with stress. Regular exercise yields physical fitness, good
mood, and well being across all ages and both sexes. The findings
are supported by psychological, physiological, including neuromuscular,
evidence11. Quality of life scores improved significantly
with condition training. With prolonged exercise training, runners
seem to become more self-sufficient and more relaxed than before running.
Jasnoske and Holmes have observed significant correlation between
aerobic capacity and greater self-assurance and reduced tension11.
However, overexercising and repetitive competition can have harmful
physical (staleness), psychological (tension, anxiety, and depression),
and social (isolation) consequences12. The interaction
of many factors--expectation, distraction, self-motivation, social
interaction and therapeutic attention-- has been suggested to be the
cause of the beneficial effects of exercise on psychological status.
Physiological effects include improved cardiovascular fitness, increased
cortical cerebral blood flow and hemispheric synchronization, and
reduced resting muscle action potential. Biochemical changes include
increased peripheral and central catecholamine levels and activation
of the opioid-hypothalmic-pituitary-adrenal axis with co-release of
beta-endorphins13,14.
| Rational nutrition
is a prerequisite for exercise.
The famous saying, Life is an exercise" illustrates the
importance of exercise. However, the best effects of exercise
on health are achieved with scientific guidance for training
with rational nutrition. For example, iron deficiency is the
main cause of anemia in Chinese children and adolescent athletes15.
Iron deficiency anemia (IDA), even in a mild form affects physical
work capacity and work efficiency16,17. In turn,
exercise affects iron absorption18 (Table 1).
The proportion of dietary heme iron of Chinese
athletes is low. The loss of iron through sweat in athletes
cannot be neglected19. A study of the affects of
IDA on erythrocytes showed that the damage was more serious
in exercised rats with IDA. Loss of the band 3 protein of erythrocyte
(RBC) membrane and the significant decrease of RBC deform-ability
index percentages in the exercised rats with IDA implicated
RBC membrane skeleton damage and a shortening of RBC life span20
(Fig 1).
|
Table 1. Effects of exercise
training on iron absorption rate of child soccer players
| Indices |
Training period
|
Non-training period
|
| Dietary 58Fe
intake during expt'l period (m g) |
221 ± 40
|
226 ± 42
|
| Oral intake of
58Fe (m g) |
923 ± 0
|
923 ± 0
|
| Total amount of
58Fe intake (m g) |
1144 ± 40
|
1148 ± 42
|
| Output of fecal
58Fe (m g) |
1056 ± 56
|
1012 ± 68
|
| Iron absorption
rate (%) |
9.1 ± 2.9*
|
l 1 .9 ± 4.7
|
(Adapted from Huo Zhou Ping and Chen Ji Di et
al18)
*Data compared to non-training period showed significance, P<
0.05
|
| Figure 1.
Effects of iron deficiency on RBC Dimax% of sedentary
and exercise rats20 |
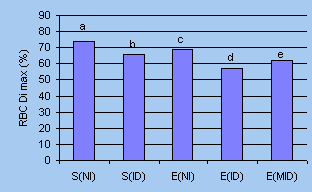
Data not sharing a common letter on
top of the columns differ significantly. S-sedentary; E- exercise;
NI- normal iron feed (iron content: 44ppm); ID- iron deficiency
feed (iron content: 4ppm); MID- mild iron deficiency feed (iron
content: 14ppm) |
| These results have provided
evidence for the impor 1000 tance of iron nutrition for athletes.
Intense exercise, with or without stress, was found to increase
serum lipid peroxide levels. Lipid peroxides accumulated in the
body are a direct cause of many diseases including age-related
disease and even aging itself. RBC malonic dealdehyde (MDA) increased
significantly after a bout of acute aerobic exercise (cycling
at heart rate 170/min on a Monark Ergometer for 60 min)21
(Table 2).
If the activities of free radical elimination enzymes such as
superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX)
increase concurrently, the appropriate defensive system is working
during exercise. Moderate exercise, especially after physical
load has been adapted, has been found to decrease blood MDA
levels and increase the free radical elimination enzyme activities21-22.
RBC MDA levels were significantly lower and SOD and GSH-PX activities
were significantly higher in elite athletes from the national
training team than in those from university because nutritional
and training status were both much better in elite athletes
(Table 3).
|
Table 2. Changes of RBC MDA
levels, RBC SOD and GSH-Px activity of athletes after a bout of
aerobic exercise
| |
RBC MDA
(nMol/gHb)
|
RBC SOD (m g/gHb)
|
RBC GSH-Px (m /gHb)
|
|
Pre-exercise
|
21.5± 0.9
|
399.3± 12.0
|
687.5± 20.3
|
|
Post-exercise
|
31.4± 1.3*
|
397.2± 13.6
|
859.5± 26.9**
|
(Adapted fr 1000 om Cao Guo Hua and Chen Ji
Di21)
*Data compared with pre-exercise, P< 0.05
**Data compared with pre-exercise, P< 0.01
|
Table 3. Comparison of MDA
levels and SOD and GSH-Px activity between elite athletes and
student athletes
| |
Elite athletes (n=30)
|
Student athletes (n=12)
|
|
Plasma MDA
(nMol/ml)
|
1.58 ± 0.04
|
1.87 ± 0.25
|
|
RBC MDA
(nMol/gHb)
|
16.26 ± 1.12
|
21.21± 0.87**
|
|
RBC SOD
(m g/gHb)
|
500 ± 20
|
408 ± 11*
|
|
RBC GSH-Px
(m g/gHb)
|
873 ± l0.6
|
657 ± 18.8**
|
**Data compared with elite athletes, P< 0.001
* P< 0.05
(Adapted from Cao Guo Hua and Chen Ji
Di23)
It was also found that RBC SOD activity was
significantly correlated with RBC zinc levels (r = 0.495, P
< 0.05)23. Zinc deficiency leads to an increase
of free radical ge 1000 neration, lipid peroxidation, and a
depressed hepatic CuZn-SOD activity in both sedentary and exercised
mice. Exercise strengthened the free radical elimination but
zinc deficiency abolished the exercise induced adaptation24
(Table 4,5).
Study of the effect of exercise and zinc deficiency
on murine immune function showed that zinc deficiency and food
restriction are both harmful to T-cell function. There is a
decrease in splenic mononuclear cell (MNC) number, and a reduction
in proliferation response of splenic cell to Con A and of IL-2
secretion25 (Table 6). Exercise exacerbated the damage
caused by zinc deficiency and food restriction. Zinc repletion
only partially repaired the damage of immune function induced
by zinc deficiency.
A three year follow-up study of a group of female
gymnasts on long term weight control diets showed detrimental
effects including growth retardation, menstrual disturbances,
malnutrition, mental stress, and muscular weakness. The weight
and height of the gymnasts were much smaller and shorter respectively
than similarly aged urban girls. This effect may reflect both
diet and selection of gymnasts. The menarche was delayed by
one to two years, the average age for the observed gymnasts
to have their first menstruation was 15.6 ± 1.58 years. It was 17.8
± 0.57 years for those who had developed secondary amenorrhea
and took medicines for the retarded menstruation. Energy, protein
and minerals were in negative balance.
Diet improvements for female gymnasts included
an increase of calories to > 90% of the recommendation and
protein intake to 1.9g/ kg of weight. Increased vegetable, fruit,
and milk product intakes to ensure adequate of mineral and vitamin
status were encouraged. There were improvements in growth and
development, nutrient balance, and mental stress, with no increase
of body fat. Weight reduction should be discouraged if athletes
train normally and their body fat does not increase or is under
10% of their total weight26,27.
|
Table 4. Effects of zinc deficiency
on MDA levels of liver tissue of mice (n= 8)
| |
|
cytoplasm (nMol/mg
prot) |
mitochondria (nMol/mg
prot) |
| Zinc deficiency |
Non-exerc |
0.184± 0.017a |
0.174± 0.007a |
| |
Exerc |
0.171± 0.014a |
0.205± 0.013b |
| Pair fed |
Non-exerc |
0.093± 0.005b |
0.111+ 0.006c |
| |
Exerc |
0.123± 0.007c |
0.171± 0.010ad |
| Normal control |
Non-exerc |
0.120± 0.008c |
0.145± 0.011d |
| |
Exerc |
0.121± 0.007c |
0.148± 0.006d |
Data sharing a common letter at the upper right
does not differ significantly, P> 0.05 (Data adapted from
Cao Guo Hua and Chen Ji Di31)
Table 5. Effects of zinc deficiency on
SOD activity of mice
| |
|
Blood
(m /ml ) |
Liver cyto-plasm
(m /mg prot ) |
Liver mito-chondria
(m /mg prot ) |
| Zinc deficient |
non-exerc |
480± 15.1a |
84.9± 2.3a |
21.9± 1.6a |
| |
exerc |
513± 19.8ab |
86.6± 3.1ab |
24.9± 1.2ab |
| Pair fed |
non-exerc |
508± 21.7ab |
91.3± 2.4bc |
23.7± 0.9a |
| |
exerc |
551± 11.6bc |
98.5± 5.1c |
28.7± 1.8b |
| normal control |
non-exerc |
527± 14.3b
|
95.9± 2.4c |
22.1± 0.9a |
| |
exerc |
593± 20.0c |
119.2± 3.9c |
27.6± 1.5b |
(Adapted from Cao Guo Hua and Chen Ji Di24)
No significant differences were seen if the data share a common
letter at the upper right, P>0.05 n=8
Table 6. Effects of zinc deficiency and
exercise on murine splenic lymphocytes (X± SD)
| |
MNCx106 spleen
|
Con A (cpm)
|
IL-2 (cu/ml)
|
|
n
|
9
|
8
|
6
|
|
ZD
|
T
|
6.08± 1.85a
|
3867 ± 231a
|
3.27 ± 0.65a
|
| |
S
|
8.66± 2.75a
|
5353± l967a
|
4.08± l.86ab
|
|
PF
1000 |
T
|
16.02± 7.67ab
|
6294± l836b
|
5.32± 3.48b
|
| |
S
|
22.06± l2.79b
|
15469± 5228c
|
15.15± 5.81c
|
|
AL
|
T
|
35.9± l8.84c
|
18275± 5195c
|
32.93± 11.18 d
|
| |
S
|
43.77 ± l4.17c
|
19472± l967c
|
23.97e± l0.82
|
ZD: zinc deficien 1000 cy; PF: pair fed; AL:
ad libitum T: exercise group; S: sedentary group. Data without
a same letter differ significantly, P< 0.05
(Adapted from Feng Jian Ying and Chen Ji Di25)
|
| Figure 2.
Comparison of body fat between elite and non-elite athletes26 |
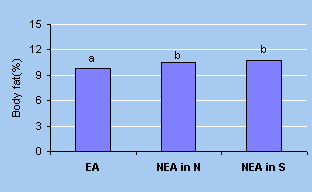
Data not sharing a common letter on the
top of the columns differ significantly EA: elite athletes from
national team; NEA in N: non-elite athletes in Northern parts;
NEA in S: non-elite athletes in Southern parts |
Exercise should begin in childhood.
Adequate exercise training with proper nutrition,
started during childhood, will produce the best health benefits. Differences
in diet, exercise, and health may affect the growth and development
stages to a certain extent. Exercise is important for skeletal development28.
Physical exercise probably induces bone mass increment by stimulating
osteogenic cells by a piezoelectric mechanism. A sedentary lifestyle
will induce bone mineral absorption due to poor mechanical stresses
acting on the bone with an increase in urinary calcium excretion.
In general, exercise during growth, creates a skeleton composed of
denser, stronger bone that is better able to withstand stress28,29.
Exercise started in childhood is also very important
in the prevention of obesity. Obesity is known to be related to an
increased risk of more than 20 diseases such as hypertension, coronary
heart disease, diabetes, hyperlipidemia, cholelithiasis, cancer, and
osteoarthritis. When several risk factors are combined, the overall
risk is many times greater. Obesity induces serious psychological
effects as well. Obese people can have less muscle mass than people
of normal weight if not active. In addition, the cardiovascular demands
of activities are greater in the obese28. Limitation of
physical activity may contribute to begin a vicious cycle. The prevention
of obesity is particularly important during periods of rapid growth,
as fat cells multiply during these times. Body fat tissues can be
enlarged by increasing either the amount of fat stored in each cell
or by increasing the number of cells in adipose tissue. The number
of fat cells seems to increase up to about 16 years of age, after
which increased fat ordinarily accumulates by increasing the size
of cells already present. Fat cell number does not decrease with weight
loss, but the size of the cells can be dramatically reduced by diet
and exercise. Obesity of childhood onset is the most severe and is
characterized by adipose tissue which contains 4-5 times the number
of fat cells as people of normal weight. Approximately 80% of obese
children remain obese as adults and thus the outcome of obesity treatment
is much more likely to be difficult and negative30. Body
fat of students who exercise regularly compared to less physically
active students is often lower and lean body mass tends to be higher31.
Percentages of body fat of elite national gymnasts were significantly
less than those of provincial athletes26 (Fig. 2).
The evidence shows that regular exercise and appropriate
diet may prevent the development of fat cells and thus be a factor
in lifelong weight control28,30. Exercise not only promotes
growth and development of bone and muscle, it also enhances the growth
of muscle strength and improves muscle coordination capacity through
the central nervous system. Thus, children and adolescent athlete
1000 s are much stronger in coping with physical load and stress than
less active children, and the onset of fatigue comes late in athletes
with physical load28,30. Therefore, the fostering of regular
exercise habits early in childhood can play an important role in the
life-long maintenance of health. Medical professionals, parents, and
educators should ensure that children grow into accomplished and secure
adults by encouraging physical activity.
Conclusions
1. The benefits of exercise are:
- Exercise may reduce the risk factors for chronic
diseases such as obesity, coronary heart disease, diabetes, and
osteoporosis.
- Exercise may improve human functional status and
metabolism.
- Exercise improves psychological status and capacity
to cope with stress.
2. Adequate physical load defined scientifically and
rational nutrition are the prerequisites for exercise. Malnutrition
such as iron or zinc deficiency, not only affects work capacity, but
also induces an increase in lipid peroxide levels and impairs immune
function. Long term food restriction is harmful for adolescent athletes
and should be discouraged.
3. The fostering of regular physical activity habits
early in childhood can play an important role in the maintenance of
health and quality of life. Physical exercise stimulates bone and
muscle development. Exercise during growth creates a skeleton composed
of denser, stronger bone and muscle mass that is better able to withstand
stress because muscle strength is improved. Exercise started in childhood
helps prevent obesity and is crucial for good mood and psychological
state.
Benefits of physical activity
on nutrition and health status: Studies in China
Chen Ji-Di
Asia Pacific J C Nutr (1995) 4,
Suppl 1

References
- James WPT. The role of nutrition and fitness in
chronic diseases. Am J Clin Nutr 1989(suppl); 49(5): 933-934.
- Rontoyannis. Diet and exercise in the prevention
of cardiovascular disease. In Simopoulos AP, ed, Nutrition and Fitness
in Health and Disease, World Rev Nutr Diet. Basel, Karger, 1993;
72: 9-22.
- Antonini FM, Vannucci A. Exercise and nutrition
in the elderly. In: Fabris F, Pernigotti L, and Ferrario E, eds,
Sedentary Life and Nutrition, New York: Raven Press, LTD, 1990;
38: 89-94.
- Ferrario E, Visentin P, Pernigotti, and Fabris
F. Metabolic modification after physical activity. In: Fabris F,
Pernigotti L, and Ferrario E, eds, Sedentary Life and Nutrition,
New York: Raven Press, LTD., 1990; 38: 95-101.
- Li Z-Y, Ding Z, Liu J-B. The dynamic observation
on cardio-pulmonary function of adolescent middle and long distance
runners during submaximum exercise. Chinese J Sports Med 1984; 3:
225-230.
- Feng H-L, Li S-J, Cheng M-Y, Li Y-Z. Effects of
8 months systematic training on cardiac function of 41 five to six
years old children. Chinese J Sports Med 1991; 10(2): 112-113.
- Yin Y-P. Effect of jogging on microcirculation
of nail fold in the aged. Chinese J Sports Med 1993; 12(4): 240-242.
- 1000 Chang W-Y, Jia S-Y, Wang J-L et al. Health
effect of 2-5 years Dao-Yin preserve skill practice on cardiovascular
system of 31 middle and old aged people. Chinese J Sports Med 1990;
9(3): 183-184.
- Zheng Y-M, He T-M, Chang L-F. Observation on serum
lipids of exercise and no exercise aged people. Chinese J Sports
Med 1992; 11(1): 57-58.
- Toshitaka T, Mitsuru H, Koji O et al. Effect of
exercise on plasma lipoprotein metabolism. In: Sato Y, Poortmans
J, Hashimoto I, Oshida Y, eds, Integration of Medical and Sports
Sciences. Med Sport Sci. Basel, Karger, 1992; 37: 430-438.
- Casper RC. Exercise and mood. In: Simopoulos AP,
Pavlou KN, eds, Nutrition and Fitness for Athletes, World Rev Nutr
and Diet. Basel, Karger, 1993; 91: 115-142.
- Morgan WP, Costill DL, Flym MG et al. Mood disturbance
following increased training in swimmers. Med Sci Sport Exerc 1988;
20: 408-414.
- Farrell PA, Gustafson A, Morgan NP, et al. Enkaphalins,
catechola-mines and psychological mood alterations: Effects of prolonged
exercise. Med Sci Sports Exerc 1987; l9: 347-353.
- Kraemer RR, Dzewaltowski DA, Blair MS, et al. Mood
alteration from treadmill running and its relationship to beta-endorphin,
corticotrophin, and growth hormone. J Sports Med Phys Fitness 1990;
30: 241-246.
- Chen J-D, Li K-J, Chen Z-M et al. Study on anemia
of athletes. Chinese J Sports Med 1990; 9(4): 193-197.
- Zheng B-Y, Yan L-Y. Effects of mild iron deficiency
anemia on children's physical work capacity. Acta Nutrimenta Sinica
1988; 10(1): 39-45.
- Li R-W, Chen X-C, Yan H-C, et al. Effects of iron
supplementation on production, efficiency of iron deficient female
textile worker. Acta Nutrimenta Sinica 1993; 15(1): 32-37.
- Chen J-D, Li K-J, Chen Z-M, et al. Study on iron
status and its related factors in adolescent athletes. Chinese J
Sports Med l990; 9(3): 140-144.
- Chen J-D, Wang J-F, Wang S-W, et al. Recommended
dietary allowances for Chinese athletes, suggestions and illustrations.
In Sato Y, Poortmans J, Hashimoto I, Oshida Y, eds, Integration
of Medical and Sports Sciences. Med Sport Sci. Basel, Karger, 1992;
37: 336-341.
- Chen J-D, Liu X-P, Tao Z-L, Wu L, Chen Z-M, et
al. Effects of iron deficiency anemia and exercise load on erythrocyte
damage in rats. In: Biochemistry of Exercise 9th International Conference,
Aberdeen; 1994: 86.
- Cao G-H, Chen J-D. The effects of one bout of acute
exercise on the free radical generation and the free radical defense
system in humans. Chinese J sports Med l991; 10(1): 1-3.
- Cao G-H, Chen J-D. The effects of swimming training
on the free radical formation and the free radical elimination in
mice. Chinese J Sports Med l991; 10(2): 65-68.
- Cao G-H, Chen J-D. Free radical generation and
elimination in athletes and their relation to zinc and copper status.
Chinese J Sports Med l991; 10(3): 132-135.
- Cao G-H, Chen J-D. Effect of zinc deficiency on
the free radical generation and the free radical elimination in
exercised mice. Chinese J Sports Med 1991; 10(4): 205-210.
- Feng J-Y, Chen J-D. Effect 9d7 of exercise and
zinc deficiency on marine immune function. Chinese J Sports Med
1994; 13(2): 88-92.
- Chen J-D, Yang Z-Y, Jiao Y, Bai R-Y, Chen Z-M,
Wu Y-Z. The nutrition and body composition of girl gymnasts during
weight control. J Sports Sci (in Chinese) 1987; 7: 22-25.
- Chen J-D. Medical problems of rapid weight and
long term weight control in athletes. In: Tsopanakis A and Poortmans
J, eds. Physiological Biochemistry of Exercise and Training, Proceedings
3rd International Course. Athens: Hellenic Sports Research Institute,
Olympic Sports Center of Athens, 1986, 257-266.
- Brooks GA. Growth and Development. In: Brooks GA,
Fahey TD, eds. Exercise Physiology, Human Bioenergetics and Its
Application. New York, Chichester, Brisbane, Toronto, Singapore:
John Wiley and Sons, Inc., 1984, 661-680.
- Campagnoli C, Belforte P, Bracci T, Isaia G. Prevention
and therapy of postmenopausal osteoporosis, role of nutrition and
physical activity. In: Fabris F, Pernigotti L, Ferrario E, eds.
Sedentary Life and Nutrition. Raven Press: 1990, 125-135.
- Lamb DR. Exercise, body composition and weight
control. In: Lamb DR, ed, Physiology of Exercise, Responses, and
Adaptations, 2nd ed, New York, London: Macmillan Publishing Company,
1984,121-134.
- Zheng SQ. Measurement of body composition and equation
for estimating the percentage of fat in college students. Chinese
J Sports Med 1984; 3(2): 76-78.

Copyright © 1995 [Asia Pacific Journal of Clinical
Nutrition]. All rights reserved.
Revised:
January 19, 1999
.
0
|